 |
| Malarial parasite, Plasmodium falciparum, in human blood. |
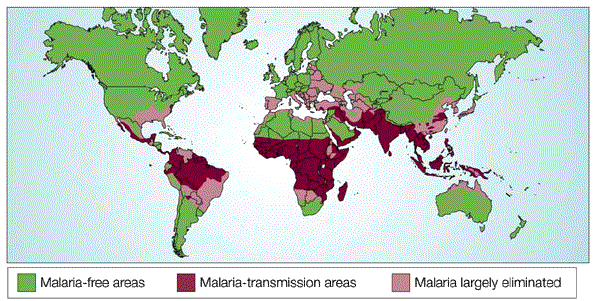
A month or so ago we were in the south of France, being eaten alive every night by mosquitoes. Fortunately, apart from the infernal itching and red blotches, we suffered little real harm because Europe is now malaria-free, but in Africa, India, Southeast Asia and South America, hundreds of millions of humans get malaria every year by being bitten by mosquitoes carrying one or other of the four species of Plasmodium parasites. About a million children die from malaria every year.
A 2008 article in Nature Education summed up how this enormous selection pressure has caused humans to evolve several different forms of resistance:
In the 1940s, J. B. S. Haldane observed that many red blood cell disorders, such as sickle-cell anemia and various thalassemias, were prominent in tropical regions where malaria was endemic (Haldane, 1949; Figure 1). Haldane hypothesized that these disorders had become common in these regions because natural selection had acted to increase the prevalence of traits that protect individuals from malaria. Just a few years later, Haldane's so-called "malaria hypothesis" was confirmed by researcher A. C. Allison, who demonstrated that the geographical distribution of the sickle-cell mutation in the beta hemoglobin gene (HBB) was limited to Africa and correlated with malaria endemicity. Allison further noted that individuals who carried the sickle-cell trait were resistant to malaria (Allison, 1954).
Allison's confirmation of Haldane's hypothesis provided the first elucidated example of human adaptation since natural selection had been proposed a century earlier. Today, this and other demonstrations of natural selection help point researchers toward biological mechanisms of resistance to infectious disease. Moreover, such examples also shed light on the ways in which pathogens rapidly evolve to remain agents of human morbidity and mortality.
Selection for Malaria Resistance: A Closer Look
Since Allison and Haldane's work, the action of natural selection on genetic resistance to malaria has been shown in a multitude of contexts (Kwiatkowski, 2005). Indeed, the sickle-cell variant (i.e., the HbS allele) has been identified in four distinct genetic backgrounds in different African populations, suggesting that the same mutation arose independently several times through convergent evolution. Beyond HbS, other distinct mutations in the HBB gene have generated the HbC and HbE alleles, which arose and spread in Africa and in Southeast Asia, respectively.
The various HBB alleles aren't alone in offering protection against malaria, however. The geographic distributions of several other red blood cell disorders, including a-thalassemia, G6PD deficiency, and ovalocytosis, correlate to malaria endemicity, and the diseases also are linked to malaria resistance. An even more striking worldwide geographical difference exists for a mutation in the Duffy antigen gene (FY), which encodes a membrane protein used by the Plasmodium vivax malaria parasite to enter red blood cells. This mutation disrupts the protein, thus conferring protection against P. vivax malaria, and it occurs at a prevalence of 100% throughout most of sub-Saharan Africa yet is virtually absent outside of Africa. Moreover, through convergent evolution, an independent mutation in FY that decreases this gene's expression has also become prevalent in Southeast Asia.
So, why has malaria exerted such strong selective pressure? Scientists now know the answer. Malaria is arguably one of the human population's oldest diseases and greatest causes of morbidity and mortality. Research indicates that the malaria-causing parasite Plasmodium falciparum has occurred in human populations for approximately 100,000 years, with a large population expansion in the last 10,000 years as human populations began to move into settlements (Hartl, 2004). P. falciparum, together with the other malaria species, P. vivax, P. malariae, and P. ovale, infects hundreds of millions of people worldwide each year, and kills more than 1 million children annually (World Health Organization, 2000). Because this disease is so devastating, humans have had to evolve adaptive traits to survive in the face of this infectious condition over the past few millennia (Kwiatkowski, 2005).
Sabeti, P. (2008) Natural selection: uncovering mechanisms of evolutionary adaptation to infectious disease. Nature Education 1(1):13
What this illustrates is how adaptive evolution doesn't just happen as some sort of random, haphazard process the way creationist pseudoscience frauds like to pretend science claims, but is driven by the environment. This is, to put it simply, the environment gives the context and the meaning to changes in the genome. A mutation is not beneficial or deleterious except in the context given to it by its environment. In the presence of a high incidence of malaria, even a mutation which can give rise to a life-limiting disease can be beneficial. In fact, the sickle-cell gene appears to have arisen several times by different mutations, showing the power of natural selection to increase the frequency of a beneficial gene in the gene pool.
 |
| Distribution of sickle-cell gene. |
 |
| Historical distribution of malaria. |
Under normal circumstances, removal of harmful genes from the gene pool like this should lead to their elimination from the gene pool altogether, only being replaced by new mutations. In the presence of malaria, however, removal of genes from the gene pool by natural selection against carriers of two recessive genes is balanced by natural selection favouring carriers on one mutant gene and one normal one.
As the number of carriers increases in the gene pool however, the probability of an individual being born with two mutant genes also increases because the chances of two carriers breeding together increases, so the removal rate increases accordingly. The result is a dynamic equilibrium or balance determined by the incidence of malaria, which is why the incidence of sickle-cell disease very closely follows the incidence of malaria in the world.
As was noted in the above article from Nature Education, sickle-cell is not the only way humans have evolved a partial resistance to malaria. The problem is that several of these, like thalassemia, also cause disorders in humans, so a similar balance is achieved. The malaria organisms, P. vivax, P. falciparum, P. malariae, and P. ovale are also evolving - which is why there are four different species. Natural selection doesn't favour any one species over another and is just as capable of leading to the evolution of Plasmodium species better able to infect and parasitise humans (which is how they evolved in the first place) as it is of leading to the evolution of humans with improved resistance to Plasmodium.
This is the classic evolutionary arms race which neither side can opt out of but from which, in the long term, neither side benefits despite the investments made. This is how we know there is no morality, let alone any intelligence-based morality subscribing to human notions of right and wrong and which looks favourably on humans in nature. I'm afraid the notion of a nice cosy Universe designed especially for humans just doesn't equate to the facts of nature.
Of course, any creationist who survived this far without having to hide from those facts to maintain their self-important, rose-tinted view of reality, can always convince me otherwise. All they have to do is explain sickle-cell disease, the malaria parasites and the coincidence of the distribution of both being almost identical, in terms of intelligent design by an omnibenevolent, intelligent designer. Why did
In your own time...

No comments:
Post a Comment
Obscene, threatening or obnoxious messages, preaching, abuse and spam will be removed, as will anything by known Internet trolls and stalkers, by known sock-puppet accounts and anything not connected with the post,
A claim made without evidence can be dismissed without evidence. Remember: your opinion is not an established fact unless corroborated.