Wooded grasslands flourished in Africa 21 million years ago – new research forces a rethink of ape evolution
Scientists are changing their mind about when and why the ancestors of hominins evolved to walk upright - but it's no comfort for creationists, as it all happened 21 million years before they believe Earth existed and only needs the standard Theory of Evolution by Natural Selection to explain it.
There is no doubt about how it evolved. Needless to say, as always, the paleoanthropologists found nothing that needed magic or a supernatural deity to explain it.
The scientists have published their findings in two papers in Science, one by a team led by Daniel Peppe, Associate Professor, Department of Geosciences, Baylor University, and the second led by Laura M. MacLatchy, Professor of Anthropology, University of Michigan.
Three of Laura MacLatchy's team have written about their research in The Conversation. Their article is reprinted here under a Creative Commons license, reformatted for stylistic consistency:

Wooded grasslands flourished in Africa 21 million years ago – new research forces a rethink of ape evolution

An ape that lived 21 million years ago was used to a habitat that was both grassy and wooded.
Corbin Rainbolt
Laura M. MacLatchy, University of Michigan; Dan Peppe, Baylor University, and Kieran McNulty, University of Minnesota
Human evolution is tightly connected to the environment and landscape of Africa, where our ancestors first emerged.
According to the traditional scientific narrative, Africa was once a verdant idyll of vast forests stretching from coast to coast. In these lush habitats, around 21 million years ago, the earliest ancestors of apes and humans first evolved traits – including upright posture – that distinguished them from their monkey cousins.
But then, the story went, global climates cooled and dried, and forests began to shrink. By about 10 million years ago, grasses and shrubs that were better able to tolerate the increasingly dry conditions started to take over eastern Africa, replacing forests. The earliest hominins, our distant ancestors, ventured out of the forest remnants that had been home onto the grass-covered savanna. The idea was that this new ecosystem pushed a radical change for our lineage: We became bipedal.
For a long time, researchers have linked the expansion of grasslands in Africa to the evolution of numerous human traits, including walking on two legs, using tools and hunting.
Despite the prominence of this theory, mounting evidence from paleontological and paleoclimatological research undermines it. In two recent papers, our multidisciplinary team of Kenyan, Ugandan, European and American scientists concluded that it is time finally to discard this version of the evolutionary story.
A decade ago, we began what, at the time, was a unique experiment in paleoanthropology: Several independent research teams joined together to build a regional perspective on the evolution and diversification of early apes. The project, dubbed REACHE, short for Research on Eastern African Catarrhine and Hominoid Evolution, was based on the premise that conclusions drawn from evidence across many locations would be more powerful than interpretations from individual fossil sites. We wondered whether previous researchers had missed the forest for the trees.
An ape in Uganda 21 million years ago
Based on the lifestyle of apes alive today, scientists have hypothesized that the very first ones evolved in dense forests, where they successfully fed on fruit, thanks to a few key anatomical innovations.
Chimpanzees move with an upright posture.
But was this scenario true for the earliest apes? A 21 million-year-old site in Moroto, Uganda, became an ideal place to investigate this question. There our REACHE team discovered teeth and other remains belonging to Morotopithecus, the oldest ape for which scientists have found fossils from the cranium, teeth and other parts of the skeleton.
Two bones in particular helped us understand how this species moved. A lower backbone found decades ago and curated by the Uganda National Museum had already been noted for its bony attachments for back muscles, indicating that Morotopithecus had a stiff lower back, good for climbing upright in the trees.
A discovery of our own confirmed this climbing behavior in a major way. At Moroto we found a fossil ape thigh bone that is short but strong, with a very thick shaft. This kind of bone is characteristic of living apes and helps them climb up and down trees with a vertical torso.

Three fossilized bones from Morotopithecus: a vertebra, part of a jaw and a femur.
L. MacLatchy and J. Kingston
Such inconsistencies between our evidence and the traditional narrative of ape origins led us to question other assumptions: Did Morotopithecus live in a forested habitat at all?
The environment at Moroto
To figure out Morotopithecus’ habitat, we studied the chemistry of fossil soils – called paleosols – and the microscopic remains of plants they contain in order to reconstruct the ancient climate and vegetation at Moroto.
Trees and most shrubs and nontropical grasses are classified as C₃ plants, based on the type of photosynthesis they perform. Tropical grasses, which rely on a different photosynthetic system, are known as C₄ plants. Importantly, C₃ plants and C₄ plants differ in the proportions of the various carbon isotopes they take in. That means carbon isotope ratios preserved in the paleosols can tell us the composition of the ancient vegetation.
We measured three distinct carbon isotope signatures, each providing a different perspective on the plant community: carbon resulting from decomposition of vegetation and soil microbes; carbon resulting from plant waxes; and calcium carbonate nodules formed in soils through evaporation.
Although each proxy gave us slightly different values, they converged on a single remarkable story. Moroto was not a closed forest habitat but rather a relatively open woodland environment. What’s more, we found evidence of abundant C₄ plant biomass – tropical grasses.
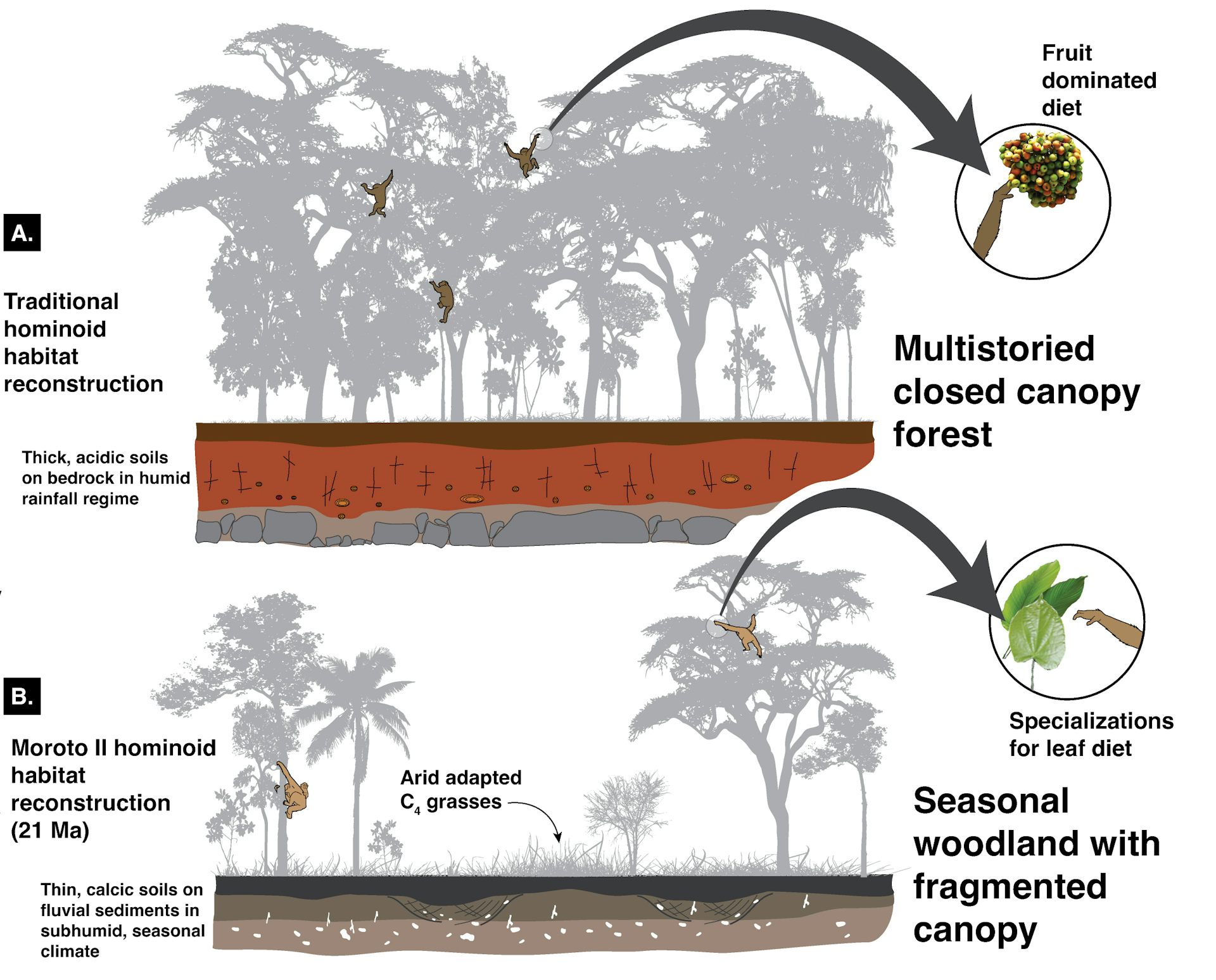
(A) Forested ecosystem traditionally believed to be the habitat of early apes, which ate fruit at the ends of tree branches, compared with (B) new perspective of grassy woodland ecosystem reconstruction, where early apes lived in open habitats and fed on leaves.
Figure modified with permission from MacLatchy et al., Science 380, eabq2835 (2023)
Our colleagues Caroline Strömberg, Alice Novello and Rahab Kinyanjui used another line of evidence to corroborate the abundance of C₄ grasses at Moroto. They analyzed phytoliths, tiny silica bodies created by plant cells, preserved in the paleosols. Their results supported an open woodland and wooded grassland environment for this time and place.
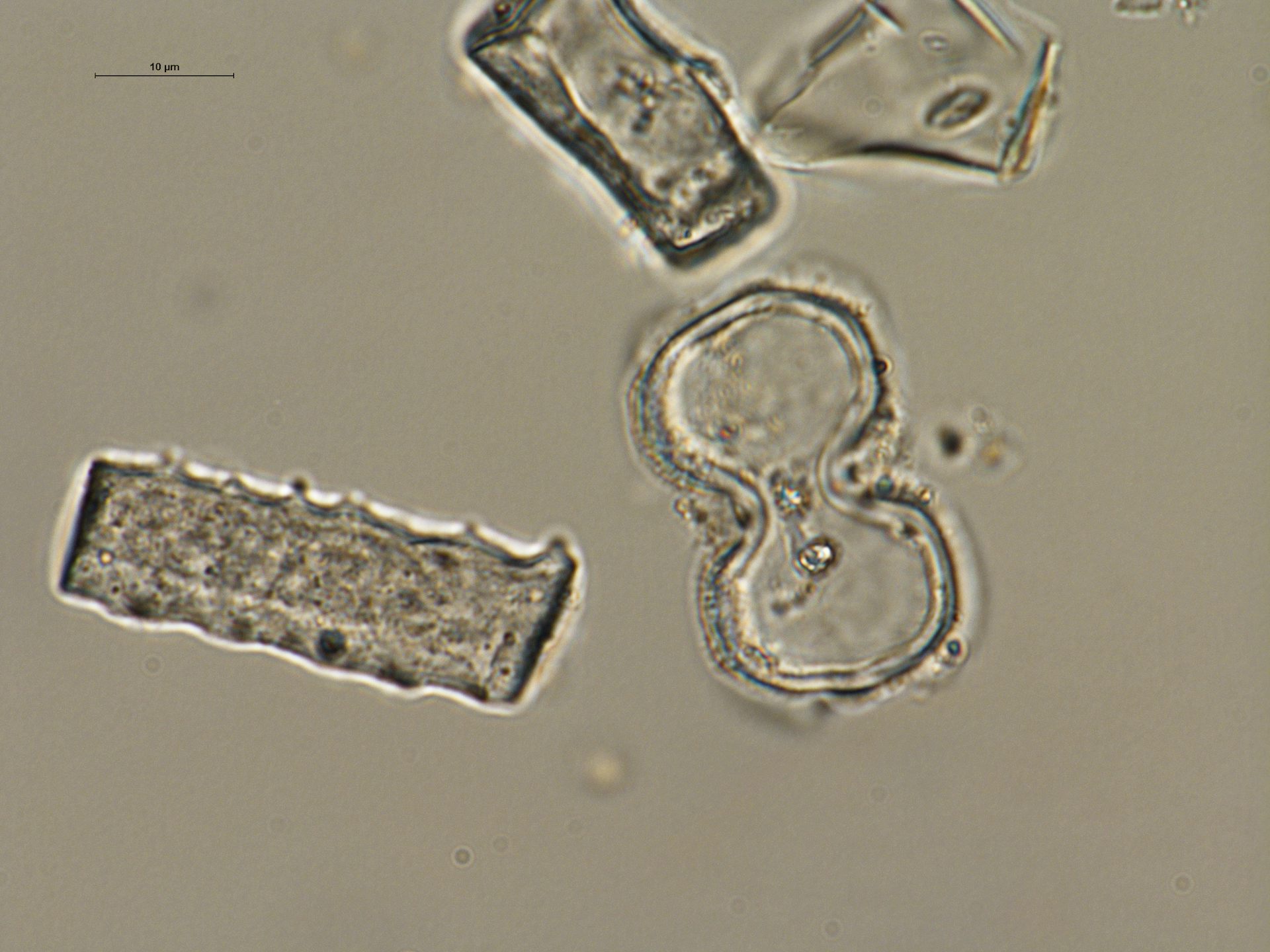
Example of typical grass phytoliths, extracted from paleosol at one of the sites, some of which indicate the presence of C₄ grass.
Alice Novello
A new, regional view of early ape habitats
Through the REACHE project, we applied the same approach to reconstruct habitats at eight other fossil sites in Kenya and Uganda, ranging in age from around 16 million to 21 million years old. After all, Morotopithecus is only one of several apes that lived during this time period.
To our surprise, we discovered that the ecological signal measured at Moroto was not unique. Instead, it was part of a broader pattern in eastern Africa during this time.
Our isotopic proxies at each fossil site contributed two significant revelations. First, vegetation types ranged from closed canopy forests to open wooded grasslands. And second, every site had a mixture of C₃ and C₄ vegetation, with some locations having a high proportion of C₄ grass biomass. Phytoliths from the same paleosols again corroborated that abundant C₄ grasses were present at multiple sites.
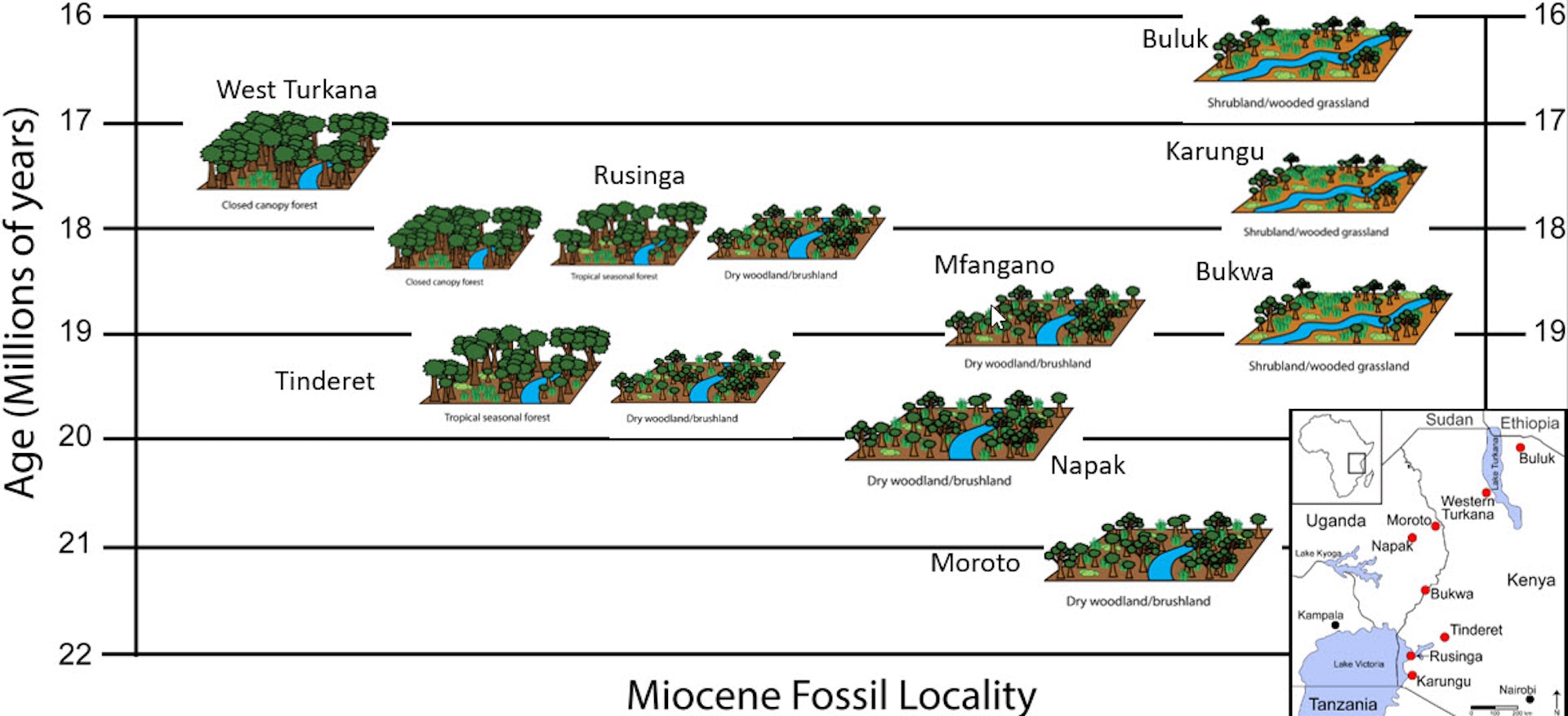
Paleoenvironments for the nine fossil sites analyzed range from closed canopy forest to more open wooded grassland environments. Inset map shows the geographic location of sites in eastern Africa.
Dan Peppe
Because the timing of the assembly of Africa’s grassland habitats underlies many evolutionary hypotheses, our discovery that they existed much earlier than expected calls for a recalibration of those ideas.
Regarding human origins, our study adds to a growing body of evidence that our divergence from apes – in anatomy, ecology, behavior – cannot be simply explained by the appearance of grassland habitats. Nevertheless, we cautiously remind ourselves that hominin evolution unfolded over many millions of years. It is almost certain that the vast and majestic grasslands of Africa played an important role in some of the many steps along the path to becoming human.
Laura M. MacLatchy, Professor of Anthropology, University of Michigan; Dan Peppe, Associate Professor of Geosciences, Baylor University, and Kieran McNulty, Professor of Anthropology, University of Minnesota
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Abstract
The assembly of Africa’s iconic C4 grassland ecosystems is central to evolutionary interpretations of many mammal lineages, including hominins. C4 grasses are thought to have become ecologically dominant in Africa only after 10 million years ago (Ma). However, paleobotanical records older than 10 Ma are sparse, limiting assessment of the timing and nature of C4 biomass expansion. This study uses a multiproxy design to document vegetation structure from nine Early Miocene mammal site complexes across eastern Africa. Results demonstrate that between ~21 and 16 Ma, C4 grasses were locally abundant, contributing to heterogeneous habitats ranging from forests to wooded grasslands. These data push back the oldest evidence of C4 grass–dominated habitats in Africa—and globally—by more than 10 million years, calling for revised paleoecological interpretations of mammalian evolution.
Daniel J. Peppe et al.
Oldest evidence of abundant C4 grasses and habitat heterogeneity in eastern Africa.
Science 380, 173-177 (2023).DOI:10.1126/science.abq2834
© 2023 The Authors. Published by American Association for the Advancement of Science.
Reprinted with kind permission under license #5613140202961
Structured Abstract
INTRODUCTION
Inherent in traditional views of ape origins is the idea that, like living apes, early large-bodied apes lived in tropical forests. In response to constraints related to locomoting in forest canopies, it has been proposed that early apes evolved their quintessential upright torsos and acrobatic climbing and suspensory abilities, enhancing their locomotor versatility, to distribute their weight among small supports and thus reach ripe fruit in the terminal branches. This feeding and locomotor transition from a quadruped with a horizontal torso is thought to have occurred in the Middle Miocene due to an increasingly seasonal climate and feeding competition from evolving monkeys. Although ecological and behavioral comparisons among living apes and monkeys provide evidence for versions of terminal branch forest frugivory hypotheses, corroboration from the early ape fossil record has been lacking, as have detailed reconstructions of the habitats where the first apes evolved.
RATIONALE
The Early Miocene fossil site of Moroto II in Uganda provides a unique opportunity to test the predictions of terminal branch forest frugivory hypotheses. Moroto II documents the oldest [21 million years ago (Ma)] well-established paleontological record of ape teeth and postcranial bones from a single locality and preserves paleoecological proxies to reconstruct the environment. The following lines of evidence from Moroto II were analyzed: (i) the functional anatomy of femora and a vertebra attributed to the ape Morotopithecus; (ii) dental traits, including molar shape and isotopic profiles of Morotopithecus enamel; (iii) isotopic dietary paleoecology of associated fossil mammals; (iv) biogeochemical signals from paleosols (ancient soils) that reflect local relative proportions of C3 (trees and shrubs) and C4 (tropical grasses and sedges that can endure water stress) vegetation as well as rainfall; and (v) assemblages of phytoliths, microscopic plant-derived silica bodies that reflect past plant communities.
RESULTS
A short, strong femur biomechanically favorable to vertical climbing and a vertebra indicating a dorsostable lower back confirm that ape fossils from Moroto II shared locomotor traits with living apes. Both Morotopithecus and a smaller ape from the site have elongated molars with well-developed crests for shearing leaves. Carbon isotopic signatures of the enamel of these apes and of other fossil mammals indicate that some mammals consistently fed on water-stressed C3 plants, and possibly also C4 vegetation, in a woodland setting. Carbon isotope values of pedogenic carbonates, paleosol organic matter, and plant waxes all point to substantial C4 grass biomass on the landscape. Analysis of paleosols also indicates subhumid, strongly seasonal rainfall, and phytolith assemblages include forms from both arid-adapted C4 grasses and forest-indicator plants.
CONCLUSION
The ancient co-occurrence of dental specializations for leaf eating, rather than ripe fruit consumption, along with ape-like locomotor abilities counters the predictions of the terminal branch forest frugivory hypotheses. The combined paleoecological evidence situates Morotopithecus in a woodland with a broken canopy and substantial grass understory including C4 species. These findings call for a new paradigm for the evolutionary origins of early apes. We propose that seasonal, wooded environments may have exerted previously unrecognized selective pressures in the evolution of arboreal apes. For example, some apes may have needed to access leaves in the higher canopy in times of low fruit availability and to be adept at ascending and descending from trees that lacked a continuous canopy.
Hominoid habitat comparisons.
Shown are reconstructions of a traditionally conceived hominoid habitat (A) and the 21 Ma Moroto II, Uganda, habitat (B).
Abstract
Living hominoids are distinguished by upright torsos and versatile locomotion. It is hypothesized that these features evolved for feeding on fruit from terminal branches in forests. To investigate the evolutionary context of hominoid adaptive origins, we analyzed multiple paleoenvironmental proxies in conjunction with hominoid fossils from the Moroto II site in Uganda. The data indicate seasonally dry woodlands with the earliest evidence of abundant C4 grasses in Africa based on a confirmed age of 21 million years ago (Ma). We demonstrate that the leaf-eating hominoid Morotopithecus consumed water-stressed vegetation, and postcrania from the site indicate ape-like locomotor adaptations. These findings suggest that the origin of hominoid locomotor versatility is associated with foraging on leaves in heterogeneous, open woodlands rather than forests.
Laura M. MacLatchy et al.
The evolution of hominoid locomotor versatility: Evidence from Moroto, a 21 Ma site in Uganda.
Science 380,eabq2835(2023).DOI:10.1126/science.abq2835
© 2023 The Authors. Published by American Association for the Advancement of Science.
Reprinted with kind permission under license #5613140365118
We have known for some time that bipedalism is not and never was a uniquely human characteristic - one of the unique characteristics that creationists cite as evidence that humans are a different form of life, distinct from all the others. In fact, not only did the Australopithecines walk upright like anatomically modern humans, but now it seems that upright bipedalism evolved much earlier - some 21 million years ago. And the evidence shows that this was not the result of magic by an invisible supernatural deity, devoid of any evidence, but was driven by climate change which led to fundamental environmental changes. A bog-standard evolutionary process in fact, with evidence of climate-led environmental change meshing perfectly with the evidence of anatomical changes associated with upright bipedalism.




No comments:
Post a Comment
Obscene, threatening or obnoxious messages, preaching, abuse and spam will be removed, as will anything by known Internet trolls and stalkers, by known sock-puppet accounts and anything not connected with the post,
A claim made without evidence can be dismissed without evidence. Remember: your opinion is not an established fact unless corroborated.