From Infamy to Ingenuity – Bacterial Hijack Mechanisms as Advanced Genetic Tools | John Innes Centre
Scientists working at the John Innes Centre, Norwich, Norfolk, UK., have worked out the sneaky way a bacterium converts an internal cell mechanism in plants to suit its own purpose at the expense of the host, in another example of how a parasite can zombify its host.
Creationists looking at this mechanism from the arrogant perspective that sees their own ignorant incredulity as scientific data, would conclude that it must be intelligently designed, but would then need to perform intellectual summersaults to explain why, even though their own putative creator god is the only supernatural entity capable of designing complex living organisms, something called 'Sin' also creates complex living organisms, so their omnipotent god is not responsible for parasites.
The parasitic Phytoplasma bacterium is transmitted by insects and causes diseases like Aster Yellows, significantly diminishing yields in leaf crops including oilseed rape, lettuce, carrots, grapevines, onions, and a variety of ornamental and vegetable crops worldwide. It is also responsible for the familiar 'witches' brooms' in trees in which the plant produces a proliferation of thin branches and leave.
How it does this is explained in an open access research paper in PNAS. It does it by hijacking the protein recycling function of a cell organelles, the 26S proteasome, so first a little AI background about the 26S proteasomes:
What is a 26S proteasome and what does it do in a cell? The 26S proteasome is a large protein complex found in the cells of eukaryotes, including humans. It plays a crucial role in the regulation of protein degradation, which is essential for maintaining cellular homeostasis and controlling various cellular processes. The 26S proteasome is part of the ubiquitin-proteasome system, a highly regulated and ATP-dependent pathway for targeted protein degradation. Here's a brief overview of how the 26S proteasome functions:The secret to the Phytoplasma bacterium's ability to hijack the process carried out by the proteasomes is a chemical called SAP05. How this works is explained in a John Innes Centre news release:The degradation of specific proteins by the 26S proteasome is a highly regulated and selective process, allowing cells to control the levels of various proteins and respond to changing environmental conditions. This system is critical for the removal of damaged or misfolded proteins, as well as for the regulation of key cellular processes such as cell cycle progression, signal transduction, and immune response. Dysregulation of the ubiquitin-proteasome system has been implicated in various diseases, including cancer and neurodegenerative disorders.
- Ubiquitination: The process begins with the attachment of small protein tags called ubiquitins to target proteins. This ubiquitination is usually a signal for degradation.
- Recognition and Binding: The ubiquitinated proteins are then recognized by the 19S regulatory particle of the 26S proteasome. This regulatory particle helps in the recognition, unfolding, and translocation of the substrate protein into the catalytic core.
- Catalytic Core: The 26S proteasome consists of a 20S catalytic core particle and two 19S regulatory particles at each end. The 20S core particle has proteolytic activities that degrade the unfolded proteins into small peptides.
- Protein Unfolding and Translocation: The 19S regulatory particle unfolds the ubiquitinated protein, ensuring that it can enter the catalytic core. The substrate is translocated into the interior of the 20S core particle for degradation.
- Proteolysis: Once inside the 20S core particle, the substrate protein is subjected to proteolysis, resulting in the generation of short peptide fragments.
- Release of Ubiquitin: The ubiquitin molecules are recycled and released to be reused in subsequent rounds of ubiquitination.
What do the terms '19S', '20S' and '26S' mean in this context?
The terms '19S,' '20S,' and '26S' refer to different subunits of the proteasome complex, each playing a specific role in the process of protein degradation.In summary, the 26S proteasome is composed of the 19S regulatory particle (responsible for substrate recognition and unfolding) and the 20S catalytic core particle (responsible for proteolysis). The numbers 19, 20, and 26 indicate the approximate molecular masses in Svedberg units, a measure of sedimentation rate used to characterize the size of biological macromolecules in a centrifugal field.
- 19S Regulatory Particle:
- The '19S' refers to the 19 subunits that make up the regulatory particle of the proteasome.
- The 19S regulatory particle is responsible for recognizing and binding to ubiquitinated proteins, helping in their unfolding, and guiding them into the catalytic core of the proteasome for degradation.
- It contains ATPase subunits that provide the energy required for the unfolding and translocation of the substrate protein.
- 20S Catalytic Core Particle:
- The '20S' refers to the 20 subunits that constitute the catalytic core particle of the proteasome.
- The 20S core particle is where the actual proteolysis takes place. It has three types of proteolytic activities: chymotrypsin-like, trypsin-like, and caspase-like activities.
- The catalytic core degrades the unfolded protein substrate into smaller peptide fragments.
- 26S Proteasome:
- The '26S' proteasome is the complete and functional proteasome complex, consisting of the 19S regulatory particle and the 20S catalytic core particle.
- The 19S regulatory particle recognizes and prepares ubiquitinated proteins for degradation, and the 20S catalytic core carries out the actual proteolysis.
- Together, the 26S proteasome is involved in the ATP-dependent degradation of ubiquitinated proteins, playing a crucial role in cellular protein quality control and regulation.
Researchers have uncovered the intricate molecular mechanism used by parasitic phytoplasma bacteria, known for inducing ‘zombie-like’ effects in plants. This detailed revelation opens new horizons for groundbreaking applications in biotechnology and even in biomedicine.
The team led by Professor Saskia Hogenhout at the John Innes Centre, in partnership with The Sainsbury Laboratory, has employed X-ray crystallography to unveil the structure and functional mechanism of SAP05. This molecule plays a crucial role in bridging two distinct components inside plant cells.
The discovery sheds new light on a peculiar phenomenon in nature – mostly seen in “witches’ brooms” in which plant stems and leaves proliferate due to Phytoplasma bacteria.
This insect-transmitted bacteria triggers diseases like Aster Yellows, significantly diminishing yields in leaf crops including oilseed rape, lettuce, carrots, grapevines, onions, and a variety of ornamental and vegetable crops worldwide.
Previous research by the Hogenhout group revealed how the bacterial protein SAP05 is able to manipulate plants by hijacking molecular machinery called the proteasome.
The proteasome breaks down and recycles proteins that are no longer required inside plant cells.
SAP05 hijacks this process, causing proteins which regulate growth and development to be dispensed into a molecular recycling centre known as the 26S proteasome.
This latest research focusses on how this happens at the structural level. SAP05 effectively disrupts the molecular recycling pathway, serving as a scaffold that connects its two cellular targets: a transcription factor and the proteasome.
Fascinatingly, SAP05 binds in a manner that enables it to ‘lift the dustbin lid’, selectively disposing of developmental proteins, while strategically preserving functions vital for the survival of its plant host.
Usually in plants, in fact across all multicellular organisms, this recycling of proteins in the proteasome is dependent on a molecule called ubiquitin. By short-circuiting this process SAP05 provides a new way of carrying out the essential task of protein degradation which serves its own parasitic purpose and is completely independent of ubiquitin.We now know the structure of this complex and how the protein binds to two cellular components to create a short circuit. Whilst SAP05 allows itself to get involved in the plant, it does not disrupt other important processes. It is so amazing to see evolution crystalized in this way.
Professor Saskia A. Hogenhout, co-corresponding author
Group leader at the John Innes Centre
Department of Crop Genetics
John Innes Centre, Norwich Research Park, Norwich, UK.
The discovery presents some intriguing possibilities. The researchers were struck by the sophisticated ingenuity of SAP05, a master manipulator, and its promising applications in biotechnology.It was so exciting to see that this molecule SAP05 had two sides, one side binding to the transcription factor and the other binding to the 26S proteasome. It’s very smart.
Dr Qun Liu, first author
Department of Crop Genetics
John Innes Centre, Norwich Research Park, Norwich, UK.
By understanding how this bacterial mechanism interacts with cells at a structural level, the researchers can now use this knowledge to engineer SAP05-like molecules which could be repurposed to remove unwanted proteins such as pathogen effectors or viruses, with an impact on therapeutics, research and in agriculture.
A highly technical explanation is given in the team's open access paper in PNAS:
SignificanceI've deliberately included lots of technical details here to give creationists a choice - they can either stand open-mouthed in ignorant awe at the brilliance of their putative designer god, to come up with something this complex in its design but simple in its operation. But the catch is, this is an example of the sheer malevolence of any designer that could come up with something like this for the sole purpose of harming plants and ruining crops.
This study reveals the structure–function relationships and mechanisms of SAP05 bacterial effectors in a unique protein degradation process. Crystal structures demonstrate that SAP05 acts as a scaffold, facilitating efficient substrate degradation by bringing together the substrate and the 26S proteasome receptor Rpn10. Substrate degradation triggered by direct interaction of SAP05 and a specific 26S proteasome component is independent of ubiquitination, distinct from PROteolysis-TArgeting Chimeras (PROTAC) and other similar systems. This finding can guide the design of SAP05-like molecules for a protein degradation technology with significant potential in biotechnology and biomedicine. It also emphasizes the capacity of proteasome subunits to initiate protein degradation, promoting the advancement of alternative approaches that bypass ubiquitination.
Abstract
In eukaryotes, targeted protein degradation (TPD) typically depends on a series of interactions among ubiquitin ligases that transfer ubiquitin molecules to substrates leading to degradation by the 26S proteasome. We previously identified that the bacterial effector protein SAP05 mediates ubiquitin-independent TPD. SAP05 forms a ternary complex via interactions with the von Willebrand Factor Type A (vWA) domain of the proteasomal ubiquitin receptor Rpn10 and the zinc-finger (ZnF) domains of the SQUAMOSA-PROMOTER BINDING PROTEIN-LIKE (SPL) and GATA BINDING FACTOR (GATA) transcription factors (TFs). This leads to direct TPD of the TFs by the 26S proteasome. Here, we report the crystal structures of the SAP05–Rpn10vWA complex at 2.17 Å resolution and of the SAP05–SPL5ZnF complex at 2.20 Å resolution. Structural analyses revealed that SAP05 displays a remarkable bimodular architecture with two distinct nonoverlapping surfaces, a “loop surface” with three protruding loops that form electrostatic interactions with ZnF, and a “sheet surface” featuring two β-sheets, loops, and α-helices that establish polar interactions with vWA. SAP05 binding to ZnF TFs involves single amino acids responsible for multiple contacts, while SAP05 binding to vWA is more stable due to the necessity of multiple mutations to break the interaction. In addition, positioning of the SAP05 complex on the 26S proteasome points to a mechanism of protein degradation. Collectively, our findings demonstrate how a small bacterial bimodular protein can bypass the canonical ubiquitin–proteasome proteolysis pathway, enabling ubiquitin-independent TPD in eukaryotic cells. This knowledge holds significant potential for the creation of TPD technologies.
 Fig 1
Fig 1
Crystal structures of SAP05–SPL5ZnF and SAP05–Rpn10vWA complexes revealing the bimodular architecture of SAP05. (A) Cartoon model illustrating the fold of the SAP05 effector. α-helices, β-sheets, and loops are indicated in cyan, red, and blue, respectively. (B) Amino acid sequence of SAP05 highlighting the locations of secondary structures (shown in A, color matched) and SPL5ZnF (purple) and Rpn10vWA interacting residues (orange) (shown in C–F, color-matched). SPL5ZnF, zinc-finger domain of SPL5 transcription factors. Rpn10vWA, von Willebrand factor type A domain of Rpn10 ubiquitin receptor. (C) Crystal structure of the SAP05–SPL5ZnF complex (PDB 8PFC). The Zn2+ ions bound to the two ZnF domains are shown in gray. (D) Crystal structure of the SAP05–Rpn10vWA complex (PDB 8PFD). In (C) and (D), the dashed lines indicate the interactions between the residues from both components. (E) Interfaces of SAP05 showing the loop surface (purple) and sheet surface (orange) residues that bind to SPL5ZnF and Rpn10vWA, respectively. (F) Gel filtration chromatograms of SAP05, SPL5ZnF, and Rpn10vWA and binary and ternary complexes of these proteins. The Coomassie-stained protein SDS-PAGE gel shows the presence of the three proteins in the SPL5ZnF–SAP05–Rpn10vWA ternary complex—see SI Appendix, Fig. S1, for the other complexes. Elution volumes are indicated at the bottom of each peak with the same colors. (G) Hypothetical ternary structure of SPL5ZnF–SAP05–Rpn10vWA obtained by superimposing the crystal structures of SAP05–SPL5ZnF and SAP05–Rpn10vWA complexes.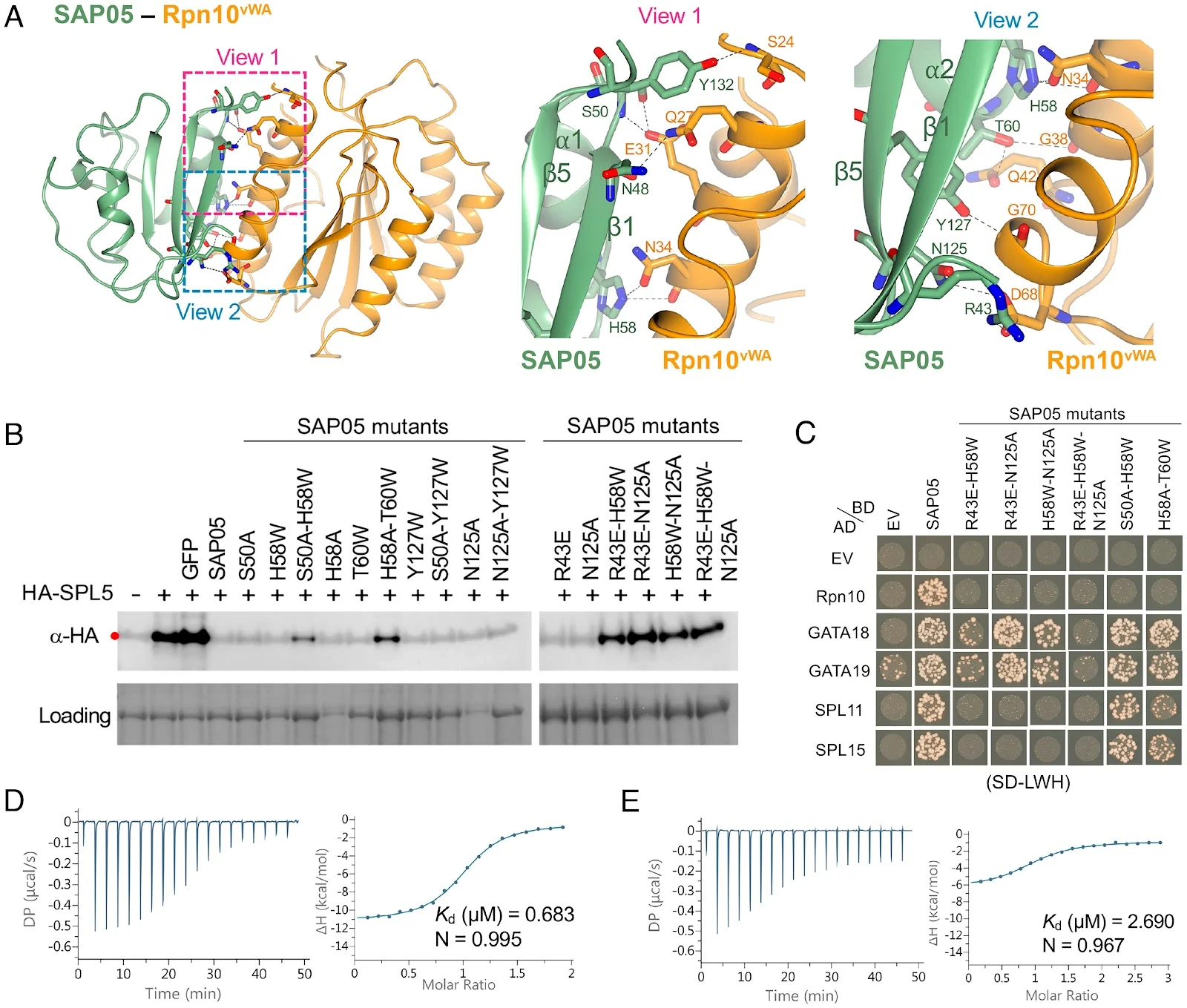 Fig 3.
Fig 3.
SAP05 β-sheet surface binds to Rpn10vWA. (A) Close-up views of SAP05–Rpn10vWA interaction interface showing the residues involved in complex formation. Left, overview of the interacting interface with two dashed squares displaying the areas for enlarged view. Middle, the enlarged view 1 of the top part in the interface. Right, the enlarged view 2 of the lower part in the interface. (B) Western blot analysis for SPL5 degradation with SAP05 wild-type and mutants in A. thaliana protoplasts. GFP, green fluorescent protein control. HA, hemagglutinin. Protein loading was visualized using Amido Black staining. (C) Y2H assay to test interactions of SAP05 and its mutants with A. thaliana Rpn10 and GATA and SPL TFs. EV, empty vector control. AD, GAL4-activation domain. BD, GAL4-DNA binding domain. SD-LWH, triple dropout medium lacking leucine, tryptophan, and histidine. See SI Appendix, Fig. S6B, for yeast growth on SD-LW medium. (D and E) ITC titrations of the SAP05–Rpn10vWA complex with SPL5ZnF (D) and SAP05H58A-T60W with SPL5ZnF (E). Left panels show heat differences upon interaction and right panels show integrated heats of injection (•) and the best fit to a single site binding model using MicroCal PEAQ-ITC analysis software. See SIAppendix, Fig. S5, for more ITC repeats and thermodynamic parameters. Fig 2.
Fig 2.
SAP05 loop surface interacts with SPL5ZnF. (A) Close-up views of SAP05–SPL5ZnF interaction interface from front (Left) and back (Right). The amino acid residues mediating electrostatic interactions are labeled. The Zn2+ ions are displayed as gray spheres. (B) Electrostatic potential surface view of SAP05 and SPL5ZnF during complex formation showing that the interacting interface is predominantly electronegative (red) in SAP05 and electropositive (blue) in SPL5ZnF. (C) ITC experiment showing direct physical binding of SAP05 and SPL5ZnF. (D) Western blot analysis of proteasomal degradation of SPL5 in the presence of wild-type or mutant SAP05 in N. benthamiana leaves. (E) Yeast two-hybrid (Y2H) assay to test interactions of SAP05 and its mutant versions with A. thaliana Rpn10 and GATA and SPL TFs. EV, empty vector control. AD, GAL4-activation domain. BD, GAL4-DNA binding domain. SD-LWHA, quadruple dropout medium lacking leucine, tryptophan, histidine, and adenine. Yeast growth on SD-LW medium is shown in SI Appendix, Fig. S6A. (F) Western blot analysis for GATA19 degradation in the presence of SAP05 D66A or N77R mutants in N. benthamiana leaves. In (D) and (F), red dots indicate the expected sizes of the transiently expressed proteins. HA, hemagglutinin. Protein loading was visualized using Ponceau S staining. (G) ITC titrations of three SAP05 mutants (D66A, D66E, and N77R) with SPL5ZnF. N.B., not binding. In (C) and (G), the top panels show heat differences upon interaction, and lower panels show integrated heats of injection (•) and the best fit to a single site binding model using MicroCal PEAQ-ITC analysis software. See SIAppendix, Fig. S5, for more ITC repeats and thermodynamic parameters. Fig 4.
Fig 4.
Steric clash at the SAP05 loop 3 region prevents SAP05 binding to human and yeast vWA domains. The SAP05–AtRpn10vWA complex structure was aligned with the vWA domain structures of human Rpn10 (PSMD4, PDB 6MSD) and yeast (S. cerevisiae) Rpn10 (PDB5LN1) to compare their configurations. The dashed squared box on the top left shows the position of loop 3. Box on the top right, sequence alignment of vWA sequences from A. thaliana Rpn10 (Uniprot ID: P55034); HsRpn10, H. sapiens Rpn10 (Uniprot ID: Q5VWC4); ScRpn10, S. cerevisiae Rpn10 (Uniprot ID: P38886). Fig 5.
Fig 5.
SAP05 interaction with the Rpn10vWA domain has no hindrance on the plant 26S proteasome. (A) Structural superimposition of SAP05–Rpn10vWA on the spinach 26S proteasome (PDB 7QVE and PDB 8AMZ). The dashed square box shows the parts with superimposition and is enlarged to show the surface (Right Top) and cartoon (Right Bottom) view of the SAP05–Rpn10vWA complex. (B) Position of Rpn10vWA residues interacting with SAP05 (green) and subunits of the 26S proteasome (pink).
This gives them the choice they can never be induced to make: is this intelligently designed and so evidence of malevolence, or did it evolve, so absolving their beloved designer of any blame?
Hint: something this complex can't be the result of 'genetic entropy' and 'devolution' (© Michael J. Behe) from something even more complex because there is nothing else like it, unless Phytoplasma bacteria were once even better at ruining crops.


No comments:
Post a Comment
Obscene, threatening or obnoxious messages, preaching, abuse and spam will be removed, as will anything by known Internet trolls and stalkers, by known sock-puppet accounts and anything not connected with the post,
A claim made without evidence can be dismissed without evidence. Remember: your opinion is not an established fact unless corroborated.