
Imagine you're in charge of an invading army laying siege to your enemy's outer defences. How do you neutralise them?
One way would be to send a small group of soldiers, packed with high explosives and deadly toxins on a kamikaze mission into the defences, with instructions to detonate their explosives and so spread the toxins when inside to destroy the defences and kill the defenders. You could improve on that by removing any temptation the suicide bombers might have to not detonate their defences by automating the trigger to fire as soon as they encountered the defender.
That's exactly what a team at the Max Planck Institute for Molecular Physiology (MPI MOPH), Dortmund, Germany, have found bacteria use to gain access to their victim’s body and make their hosts sick and die. The team, led by Stefan Raunser, Director of MPI MOPH, have published their findings, open access, in Nature Microbiology. Their work is described in a MPI MOPH press release:
How a few soldier cells confer virulence to an entire bacterial population by sacrificing themselves
You suddenly feel sick - pathogenic bacteria have managed to colonize and spread in your body! The weapons they use for their invasion are harmful toxins that target the host’s defense mechanisms and vital cell functions. Before these deadly toxins can attack host cells, bacteria must first export them from their production site – the cytoplasm – using dedicated secretion systems. The group of Stefan Raunser, Director at the Max Planck Institute of Molecular Physiology, has now elucidated a so-far enigmatic, exceptional secretion mechanism, that allows the release of the gigantic Tc toxins. In a kind of kamikaze attack, a small group of so-called “soldier” bacteria, packed to the brim with toxins, release their deadly cargo by exploding in the host. Targeting such subpopulations in medical therapies could be a promising treatment strategy for diseases triggered by bacteria that are becoming increasingly resistant to antibiotics.
Once a pathogenic bacterium has entered its host, it turns on a series of defense and attack mechanisms to spread, invade and colonize deeper tissues and organs. This includes the secretion of an array of toxic proteins that subvert the host’s cellular defenses. In gram-negative bacteria, which can trigger severe infections and are becoming increasingly resistant to antibiotics, toxic proteins face the challenge of crossing several cellular barriers – belonging to both the bacteria and the host – to finally reach their destination. To this end, bacteria have developed a number of specialized secretion systems. Some can secrete a variety of toxins and are found in almost all bacteria, while others have been identified in only few bacteria. The machinery for the secretion of many smaller toxins has already been established. Not so for larger ones, like the Tc toxins produced by the notorious Yersinia bacteria, which also include pathogens that cause plague and tuberculosis.
It has remained enigmatic for decades how the huge Tc toxins reach their final destination. By obtaining the first 3D structures of a Tc toxin in our previous electron cryomicroscopy studies we could already figure out how it bypasses the last barrier, the host membrane, using a syringe-like injection mechanism. Now, we were able to complete the picture and show how these toxins overcome the three barriers separating the inside of the bacteria from its environment in a truly spectacular way.
Stefan Raunser, lead author
Department of Structural Biochemistry
Max Planck Institute of Molecular Physiology, Dortmund, Germany.
Exploding bacteria
In their recent work, Raunser and his team have applied a cutting-edge combination of several techniques to investigate the secretion of the Tc toxin YenTc produced by the insect pathogen Yersinia entomophaga, which is crucial for this bacterial species to establish an infection. The biggest challenge was to initially identify which of the known secretion machineries is used for this purpose by the bacteria. To this end, the scientists knocked out all suspected secretion systems one after the other using targeted genome editing. When none of the knockouts stopped the toxin’s release, the same technique was used to modify the toxin so that its secretion could be visualized - and this time with success. “Watching some of the bacteria literally explode to release their toxins was a real eureka moment,” says Oleg Sitsel, first author of the study. Careful proteomic analysis then finally brought to light a pH-sensitive type 10 secretion system responsible for toxin release, a class of protein export machinery that was just recently established. Subsequent cryo-electron tomographic analysis visualized the step-by-step details of how this secretion system exports cellular contents via a previously unknown lytic mode of action that overcomes the three barriers surrounding gram-negative bacteria.
Becoming a soldier cell
The scientists found that only a small specialized subset of bacterial cells produces and exports the toxins by paying the ultimate price, namely death. But what causes those cells, which the authors termed “soldier cells”, to first enlarge and produce a deadly toxin cocktail containing YenTc, then commit suicide for the benefit of their comrades? The scientists first determined that the appearance of soldier cells is temperature-, nutrient- and cell density-dependent. They then discovered a temperature-sensitive genetic switch that synchronizes the production of the toxins with the production of the secretion system, and turns “normal” cells into their soldier brethren. The mass production of toxins coupled to the cells’ enlarged size ensures that only few individuals need to be sacrificed for the greater good of the bacterial population, an extremely efficient strategy.
We suspect that normal cells turn into soldier cells upon ingestion in response to insect host nutrients. Toxin secretion is pH sensitive, which delays its release until the soldier cells reach the alkaline posterior midgut, their major theatre of operations.
This secretion strategy is unique and remarkable. The behaviour of these bacteria exhibits characteristics such as differentiation and altruism, which are reminiscent of eusocial systems. If this turns out to be a more common mechanism, we might have exposed a weak point in bacteria: specifically targeting the soldier cells could become a promising medical strategy in the fight against pathogenic bacteria, especially in times of increasing resistance to antibiotics.
Stefan Raunser
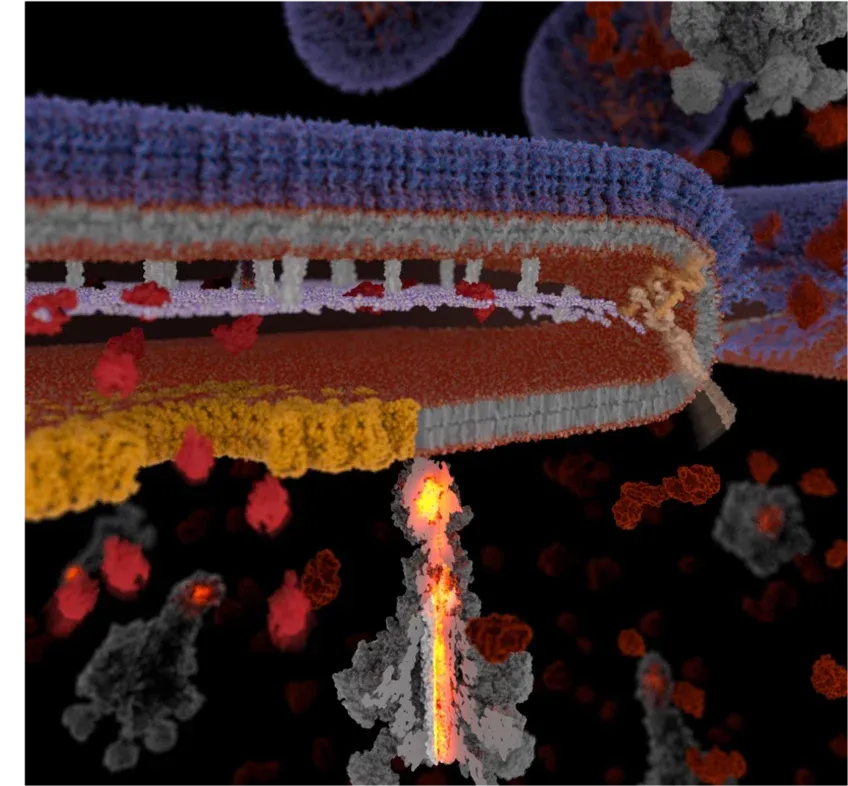
 Model for development of Soldier cells and YenTc release. Upper image: Naive cells turn into soldier cells by activation of a temperature-sensitive genetic switch in response to insect host nutrients, temperature and increased cell density. A pH-change leads to the activation of the secretion system and the release of the toxin cocktail. Lower image: YenTc release in detail.MPI MOPH
Model for development of Soldier cells and YenTc release. Upper image: Naive cells turn into soldier cells by activation of a temperature-sensitive genetic switch in response to insect host nutrients, temperature and increased cell density. A pH-change leads to the activation of the secretion system and the release of the toxin cocktail. Lower image: YenTc release in detail.MPI MOPH
AbstractTo be a commander in charge of an army using these tactics, and against an innocent 'enemy' whom you've randomly chosen to attack and massacre, you would need to be a psychopath and war-criminal to send your own soldiers to deliver deadly chemical warfare against innocent people, knowing that they won't return.
Disease-causing bacteria secrete numerous toxins to invade and subjugate their hosts. Unlike many smaller toxins, the secretion machinery of most large toxins remains enigmatic. By combining genomic editing, proteomic profiling and cryo-electron tomography of the insect pathogen Yersinia entomophaga, we demonstrate that a specialized subset of these cells produces a complex toxin cocktail, including the nearly ribosome-sized Tc toxin YenTc, which is subsequently exported by controlled cell lysis using a transcriptionally coupled, pH-dependent type 10 secretion system (T10SS). Our results dissect the Tc toxin export process by a T10SS, identifying that T10SSs operate via a previously unknown lytic mode of action and establishing them as crucial players in the size-insensitive release of cytoplasmically folded toxins. With T10SSs directly embedded in Tc toxin operons of major pathogens, we anticipate that our findings may model an important aspect of pathogenesis in bacteria with substantial impact on agriculture and healthcare.
 a, A single slice from an Ara-RoeA ΔYenLC cell tomogram, representing the pre-secretion state. Scale bar, 100 nm. n = 20 biological replicates. Inset: a fully assembled cytoplasmic YenTc holotoxin. Scale bar, 10 nm. b, Ara-RoeA ΔPepB/Rz/Rz1 cell tomogram slice, representing the state of secretion after holin action. Scale bar, 100 nm. n = 15 biological replicates. c, Ara-RoeA ΔRz/Rz1 cell tomogram slice, representing the state of secretion after endolysin action. Scale bar, 10 nm. n = 86 biological replicates. d, Ara-RoeA cell tomogram slice, representing the state of secretion after spanin action. Scale bar, 100 nm. n = 105 biological replicates. Left inset: a YenTc holotoxin secreted to the external environment. Scale bar, 10 nm. Right inset: an apparently unfused vesicle potentially derived from an area of the cell envelope that contained proteins spanning the entire cell wall. Scale bar, 10 nm. e–g, Annotated densities from the tomograms shown in a–d. Dark orange, outer membrane; purple, peptidoglycan; yellow, inner membrane; dark red, YenTc. Only those YenTc densities that could be definitively identified in these 50–100-nm-thick slices were annotated as such. h, Annotated densities from the tomogram shown in d, presented as a diagonally sectioned view to demonstrate the internal structure that arises after spanin action. Light orange, fused membranes; purple, peptidoglycan; dark red, YenTc; dark blue, potential cell envelope-spanning protein complexes.
a, A single slice from an Ara-RoeA ΔYenLC cell tomogram, representing the pre-secretion state. Scale bar, 100 nm. n = 20 biological replicates. Inset: a fully assembled cytoplasmic YenTc holotoxin. Scale bar, 10 nm. b, Ara-RoeA ΔPepB/Rz/Rz1 cell tomogram slice, representing the state of secretion after holin action. Scale bar, 100 nm. n = 15 biological replicates. c, Ara-RoeA ΔRz/Rz1 cell tomogram slice, representing the state of secretion after endolysin action. Scale bar, 10 nm. n = 86 biological replicates. d, Ara-RoeA cell tomogram slice, representing the state of secretion after spanin action. Scale bar, 100 nm. n = 105 biological replicates. Left inset: a YenTc holotoxin secreted to the external environment. Scale bar, 10 nm. Right inset: an apparently unfused vesicle potentially derived from an area of the cell envelope that contained proteins spanning the entire cell wall. Scale bar, 10 nm. e–g, Annotated densities from the tomograms shown in a–d. Dark orange, outer membrane; purple, peptidoglycan; yellow, inner membrane; dark red, YenTc. Only those YenTc densities that could be definitively identified in these 50–100-nm-thick slices were annotated as such. h, Annotated densities from the tomogram shown in d, presented as a diagonally sectioned view to demonstrate the internal structure that arises after spanin action. Light orange, fused membranes; purple, peptidoglycan; dark red, YenTc; dark blue, potential cell envelope-spanning protein complexes.
Sitsel, O., Wang, Z., Janning, P. et al.
Yersinia entomophaga Tc toxin is released by T10SS-dependent lysis of specialized cell subpopulations. Nat Microbiol (2024). https://doi.org/10.1038/s41564-023-01571-z
Copyright: © 2024 The authors.
Published by Springer Nature Ltd. Open access.
Reprinted under a Creative Commons Attribution 4.0 International license (CC BY 4.0)
Faced with this sort of evidence, many creationists prefer to look the other way and pretend it isn't there but those few who are disturbed enough by it to try to absolve their putative designer god of any responsibility for it, will normally parrot 'Sin' and 'The Fall' as though that explains anything. It's the childish excuse, "A big boy did it and ran away!"
What they seem not to be aware of is that by trying to blame something other than their supposedly omniscient, omnipotent designer god they are claiming there is some creative force that their god is powerless against and which can outwit it to circumvent the defences it supposedly designed to protect us.
So, they have three clear choices: either blaspheme and argue that their god is not supreme and can be outwitted and out-maneuvered; accept that their supposedly omnibenevolent god is actually a pestilential malevolence intent on making us sick and increasing the suffering in the world, or, accept that there is a mindless, perfectly natural, god-free process that has created pathogens and made them good at what they do. It's a curious feature of creationism that they would rather choose one of the first two options and betray their god than accept that evolution is a fact.
Illustrated by Catherine Webber-Hounslow.



No comments:
Post a Comment
Obscene, threatening or obnoxious messages, preaching, abuse and spam will be removed, as will anything by known Internet trolls and stalkers, by known sock-puppet accounts and anything not connected with the post,
A claim made without evidence can be dismissed without evidence. Remember: your opinion is not an established fact unless corroborated.