
The ancestors of all modern mammals, the synapsids, first appeared in the fossil record long before the dinosaurs. Some were vegetarian while others were the top land predators for 60 million years before being replaced by the dinosaurs. Their evolution followed the same pattern of an arms race with their prey species in which the carnivores had to get better at killing to survive.
This gives the lie to the claim of any involvement of a single intelligent designer in their evolution since no intelligent designer worthy of the name would compete with itself in an arms race in which there are no winners or losers while all the time additional complexity is needed to keep up.
Tell me all about the synapsids, please. Synapsids are a group of vertebrates that includes mammals and their extinct relatives. They are characterized by several key features, including a single temporal opening behind the eye socket, teeth set in sockets, and a more erect posture compared to earlier reptiles. Synapsids first appeared during the late Carboniferous period, about 315 million years ago, and they were a dominant group throughout much of the Permian and Triassic periods. Here are some key points about synapsids:Now a team of researchers from Bristol University, together with a colleague from the Open University, Milton Keynes, have traced the evolution of the carnivorous synapsid jaw and body size and discovered a major change in the jaw about 270 million years ago which would have been associated with a shift in the behaviour of these carnivores as they became better at killing their prey more quickly, so reducing the risk of injury.Overall, synapsids are a fascinating group of vertebrates that played a significant role in the evolutionary history of life on Earth, ultimately giving rise to the diverse and successful lineage of mammals that we see today.
- Evolutionary History: Synapsids evolved from reptilian ancestors, specifically from a group called the pelycosaurs. One of the most famous pelycosaurs is Dimetrodon, which lived during the early Permian period and is often mistakenly identified as a dinosaur. Synapsids evolved further into two main groups: the therapsids and the cynodonts.
- Therapsids: This group includes the most mammal-like synapsids. They had more advanced jaw structures, with larger temporal fenestrae (openings behind the eye socket) and specialized teeth. Therapsids diversified during the Permian period, with some species evolving into large herbivores like the iconic sail-backed Dimetrodon, while others were carnivorous predators. Towards the end of the Permian, some therapsids gave rise to the first true mammals.
- Cynodonts: Cynodonts were a subgroup of therapsids that emerged during the late Permian and diversified extensively during the Triassic period. They exhibited several mammalian characteristics, including a more upright posture, specialized teeth, and possibly hair-like structures. Some cynodonts were quite small and likely had a lifestyle similar to modern mammals, while others were larger predators.
- Mammalian Evolution: The transition from cynodonts to true mammals occurred during the Triassic period. Early mammals retained many reptilian features but also displayed characteristics that are distinctly mammalian, such as lactation and specialized jaw joints. By the end of the Triassic period, mammals had become more diverse and widespread.
- Impact on Evolutionary History: Synapsids, particularly therapsids and cynodonts, played a crucial role in evolutionary history. They were among the dominant land vertebrates during the Permian and Triassic periods, occupying various ecological niches as herbivores, carnivores, and omnivores. The extinction of many synapsid groups at the end of the Permian, possibly due to environmental changes and the effects of the Permian-Triassic mass extinction event, paved the way for the rise of dinosaurs as the dominant land vertebrates during the Mesozoic Era.
- Modern Descendants: The only surviving descendants of synapsids are mammals, which have diversified into a vast array of species occupying nearly every terrestrial and aquatic habitat on Earth. From tiny shrews to massive whales, modern mammals exhibit remarkable diversity in terms of size, shape, behavior, and ecological adaptations.
Their findings are the subject of an open access paper in Communications Biology and is the subject of a Bristol University news release:
The evolutionary success of the first large predators on land was driven by their need to improve as killers, researchers at the University of Bristol and the Open University suggest.
The forerunners of mammals ruled the Earth for about 60 million years, long before the origin of the first dinosaurs. They diversified as the top predators on land between 315–251 million years ago.
Researchers studied the jaw anatomy and body size of carnivorous synapsids, using these traits to reconstruct the likely feeding habits of these ancient predators and chart their ecological evolution through time. They found a major shift in synapsid jaw function roughly 270 million years ago linked to a significant shift in predatory behaviour that has important implications for the evolution of our earliest ancestors.
As herbivores grew larger and faster, carnivores adapted to become bigger and better predators to survive.
This finding provides important context for a key step in synapsid evolution.Earlier synapsid predators such as the famous sail backed Dimetrodon, had fairly long jaws with lots of teeth to ensure that once they ensnared their prey, it wouldn’t escape. However, we saw a shift in jaw function toward shorter jaws with greater muscle efficiency and fewer teeth that were concentrated at the front of the jaw - these were jaws adapted to deliver deep, powerful bites. The change shows that later synapsid carnivores placed more emphasis on heavily injuring and so more quickly killing their prey. Among these later synapsids were the very first sabertoothed carnivores! This change highlights that predators were facing new selective pressures from their prey.
Dr Suresh A. Singh, lead author
School of Earth Sciences.
Bristol University, Bristol, UK>
The reorganisation of synapsid jaws through this time has long been known as a big step towards the evolution of mammals. These changes don’t just make the jaw more efficient; they also mark the very earliest redevelopment of the jaw that also created the complex ear found in mammals. What drove this first step? Our study suggests that it was partly driven by ecological pressures from their prey.
Dr Armin Elsler, co-author
School of Earth Sciences.
Bristol University, Bristol, UK>This shift reflects a new dynamism in predator-prey interactions that shows that life on land was moving more quickly.The timing of the shift in jaw function corresponds with the evolution of new larger, faster herbivores that would have posed a greater challenge for predators to tackle. The risks to carnivores of getting injured or killed went up, so some synapsid carnivores became bigger, better killers to overcome these risks.
Dr Thomas L. Stubbs, so-author
School of Life
Health and Chemical Sciences
Open University, Milton Keynes, UKThe late Palaeozoic was the time when animals first began to live, eat and reproduce entirely on land. They became fully terrestrial, colonising new habitats and exploiting new resources further inland from the aquatic environments they’d previously relied on. Our findings show how the selective pressures on these early land animals changed as they became better adapted for life on land - catching another animal that can move fast and grow to larger sizes is much more difficult than catching a slippery little fish or amphibian.
Professor Michael J. Benton, co-author
School of Earth Sciences.
Bristol University, Bristol, UK>The researchers also found that synapsid carnivore morphological diversity increased following the shift, with the addition of new functional groups adapted for either faster biting speeds or even more powerful bites through the mid-late Permian – around 265-251 million years ago. By assessing how the sizes of these new carnivore species compared within different communities through time, they realised these communities may have begun to closely resemble those of modern carnivorous mammals.Predator-prey interactions are an important driver of animal behaviour today so it’s quite something to see that influence through anatomical evolution over millions of years, and find that they are potentially responsible for driving some big leaps in our own evolutionary history. It highlights how palaeontologists can use the relationship between form and function to explore how different prehistoric animals may have lived, which can tell us so much about the evolution of life on Earth.
Professor Emily J. Rayfield, co-author
School of Earth Sciences.
Bristol University, Bristol, UK>
Abstract
Terrestrial ecosystems evolved substantially through the Palaeozoic, especially the Permian, gaining much new complexity, especially among predators. Key among these predators were non-mammalian synapsids. Predator ecomorphology reflect interactions with prey and competitors, which are key controls on carnivore diversity and ecology. Therefore, carnivorous synapsids may offer insight on wider ecological evolution as the first complex, tetrapod-dominated, terrestrial ecosystems formed through the late Palaeozoic. Using morphometric and phylogenetic comparative methods, we chart carnivorous synapsid trophic morphology from the latest Carboniferous to the earliest Triassic (307-251.2 Ma). We find a major morphofunctional shift in synapsid carnivory between the early and middle Permian, via the addition of new feeding modes increasingly specialised for greater biting power or speed that captures the growing antagonism and dynamism of terrestrial tetrapod predator-prey interactions. The further evolution of new hypo- and hypercarnivorous synapsids highlight the nascent intrinsic pressures and complexification of terrestrial ecosystems across the mid-late Permian.
Introduction
Tetrapod terrestrialisation through the late Palaeozoic is a pivotal moment in Earth history, as tetrapods revolutionised life on land by adding new complexity to terrestrial trophic networks, establishing the basic relationships that still underpin terrestrial ecosystems today1,2,3,4,5. Overcoming multiple organismal and environmental constraints, tetrapods became increasingly adept on land as they evolved to better survive and exploit the resources of their new realm6,7,8,9,10. By the late Permian, this diversification produced rich communities of specialist tetrapod herbivores and carnivores, echoing the diversity of modern ecosystems11. Nonetheless, Palaeozoic ecosystems often differed structurally from modern counterparts by possessing disproportionately diverse carnivore contingents12,13,14. Such predator-rich terrestrial faunas appeared throughout the Palaeozoic and Mesozoic12,13,15,16, in contrast to more prey-rich systems that dominated the Cenozoic3,17. These differences raise the possibility of substantially differing ecological dynamics through deep time and point to the need for detailed understanding of such ancient ecosystems and their influence on tetrapod macroevolution.
A limited fossil record precludes direct analysis of Palaeozoic ecological interactions and processes18, but such interactions are a key selective pressure in evolution, driving behavioural shifts that ultimately promote phenotypic change19,20,21. Therefore, functional anatomy may provide a window onto these interactions through deep time. Carnivores, especially large macropredators, are often useful indicators of ecological change22,23 as they exert great influence over their ecosystems through antagonistic relationships with prey and competitors, which in turn, are major influences on carnivore behavioural ecology, forcing changes in habitat, diet, and foraging activity3,24,25. Palaeozoic terrestrial ecological evolution may therefore be studied using the ecomorphology of the leading terrestrial carnivores of the time: non-mammalian synapsids.
Synapsids rose quickly to prominence within the terrestrial carnivore guild during the first major radiation of terrestrial amniotes in the Late Carboniferous26, with basal, ‘pelycosaur-grade’ synapsids becoming the top terrestrial predators by the early Permian27,28. Despite extinction events at the end of the early and middle Permian that eliminated much of their diversity, synapsids maintained a monopoly on large terrestrial carnivore niches to the end of the Palaeozoic, with successive diversifications of therapsids creating rich, new carnivore communities, dominated by biarmosuchians and dinocephalians in the Guadalupian, and then gorgonopsians and therocephalians in the Lopingian2,29. Synapsid faunal dominance was ended by the Permo-Triassic Mass Extinction (PTME), allowing diapsid archosauromorphs to overtake them through the Triassic4. Synapsid monopolisation of the terrestrial carnivore guild through the late Palaeozoic offers an opportunity to study trophic ecological dynamics through the founding and development of the first complex tetrapod ecosystems on land, as well as multiple mass extinction events5,30.
By applying morphometric and macroevolutionary analytical methods including the new consensus clustering method of Singh et al.31, we reconstruct and quantitively classify the feeding ecologies of carnivorous non-mammalian synapsids through the Late Carboniferous—Early Triassic (315.2–251.2 Ma), using jaw functional morphology and body size, both of which closely relate to feeding and foraging behaviour32,33,34,35. Through a combination of geometric and traditional linear measurement-based morphometric methods31, we provide a broad assessment that captures synapsid jaw morphofunctional evolution from differing perspectives and partially mitigates the divergent impacts of phylogenetic heritage, taxonomic scaling, or methodological choices36. Even though non-mammalian synapsid jaw functionality uniquely encompasses a spectrum between reptiles and mammals unseen in extant taxa37, changes in basic functional properties (e.g., mechanical advantage or symphyseal robusticity) allow us to use absolute and relative similarities to living animals to make some rudimentary inferences and hypotheses of non-mammalian synapsid prey preferences, modes of prey capture, and consumption that can be further examined in more bespoke, future biomechanical studies. Through the identification and comparison of the distinct functional feeding groups (FFGs) of carnivorous synapsids, we extract new details on the trophic interactions of terrestrial tetrapods, revealing their ecological evolution through the Palaeozoic.Fig. 1: Synapsid carnivore jaw morpho-functional diversity. a Jaw shape morphospace. b Jaw functional characters mapped across shape morphospace. (Colour gradient reflects functional character values—see scale.) c Jaw functional morphospace, with arrows showing general functional trends. Point size represents Log10(mm) femur length. N = 122. Jaw silhouettes: 1. Smilesaurus ferox, 2. Sphenacodon ferox, 3. Secodontosaurus obtusidens, 4. Microvaranops parentis, 5. Varanodon agilis, 6. Lycideops longiceps, 7. Lobalopex mordax, 8. Ictidosaurus angusticeps, 9. Procynosuchus delaharpeae, 10. Dimetrodon milleri, 11. Vetusodon elikhulu, 12. Dinogorgon rubidgei, 13. Deuterosaurus biarmicus. 14. Mycterosaurus longiceps. BF biting force, BIA Biarmosuchia, CYN Cynodontia, DIN Dinocephalia, EOT Eothyrididae, GRG Gorgonopsia, MAMA mean anterior mechanical advantage, MAR maximum aspect ratio, MPMA mean posterior mechanical advantage, OMA opening mechanical advantage, OPH Ophiacodontidae, RAO relative articulation offset, RSL relative symphyseal length, RTL relative toothrow length, SA Symphyseal angle, SPH Sphenacodontia (non-therapsid), SR Symphyseal robusticity, THR Therocephalia, VAR Varanopidae. N = 122 taxa. Biarmosuchia, Dinocephalia and Therocephalia silhouettes by Dmitry Bogdanov (vectorized by T. Michael Keesey); all other silhouettes created by S.A.S., but some are vectorised from artwork by Felipe Alves Elias (https://www.paleozoobr.com/), available for academic use with attribution.Fig. 2: Synapsid carnivore feeding functional subgroup jaw characteristics.
a Jaw shape morphospace. b Jaw functional characters mapped across shape morphospace. (Colour gradient reflects functional character values—see scale.) c Jaw functional morphospace, with arrows showing general functional trends. Point size represents Log10(mm) femur length. N = 122. Jaw silhouettes: 1. Smilesaurus ferox, 2. Sphenacodon ferox, 3. Secodontosaurus obtusidens, 4. Microvaranops parentis, 5. Varanodon agilis, 6. Lycideops longiceps, 7. Lobalopex mordax, 8. Ictidosaurus angusticeps, 9. Procynosuchus delaharpeae, 10. Dimetrodon milleri, 11. Vetusodon elikhulu, 12. Dinogorgon rubidgei, 13. Deuterosaurus biarmicus. 14. Mycterosaurus longiceps. BF biting force, BIA Biarmosuchia, CYN Cynodontia, DIN Dinocephalia, EOT Eothyrididae, GRG Gorgonopsia, MAMA mean anterior mechanical advantage, MAR maximum aspect ratio, MPMA mean posterior mechanical advantage, OMA opening mechanical advantage, OPH Ophiacodontidae, RAO relative articulation offset, RSL relative symphyseal length, RTL relative toothrow length, SA Symphyseal angle, SPH Sphenacodontia (non-therapsid), SR Symphyseal robusticity, THR Therocephalia, VAR Varanopidae. N = 122 taxa. Biarmosuchia, Dinocephalia and Therocephalia silhouettes by Dmitry Bogdanov (vectorized by T. Michael Keesey); all other silhouettes created by S.A.S., but some are vectorised from artwork by Felipe Alves Elias (https://www.paleozoobr.com/), available for academic use with attribution.Fig. 2: Synapsid carnivore feeding functional subgroup jaw characteristics. The feeding functional subgroup jaw functional character (Supplementary Methods) distributions illustrated using violin and box plots. Functional feeding group compositions illustrated using ring plots detailing relative proportions of different taxonomic groups. Violin plots show taxon density. Box plots showing median value and upper and lower quartiles, with whisker illustrating standard deviation. Mean values indicate by black dots. Coloured arrows indicate whether values increase (red) or decrease (blue) relevant jaw functionality. N = 122. Jaw silhouettes (left to right): Varanodon agilis, Tetraceratops insignis, Dimetrodon grandis, Sauroctonus parringtoni, Smilesaurus ferox, Annatherapsidus petri, Tetracynodon darti. BIA Biarmosuchia, CYN Cynodontia, DIN Dinocephalia, EOT Eothyrididae, GRG Gorgonopsia, MAMA mean anterior mechanical advantage, MAR maximum aspect ratio, MPMA mean posterior mechanical advantage, OMA opening mechanical advantage, OPH Ophiacodontidae, RAO relative articulation offset, RSL relative symphyseal length, RTL relative toothrow length, SA Symphyseal angle, SPH Sphenacodontia (non-therapsid), THR Therocephalia, VAR Varanopidae. N = 122 taxa. Biarmosuchia, Dinocephalia and Therocephalia silhouettes by Dmitry Bogdanov (vectorized by T. Michael Keesey); all other silhouettes created by S.A.S., but some are vectorised from artwork by Felipe Alves Elias (https://www.paleozoobr.com/), available for academic use with attribution.Fig. 3: The ecofunctional focus of synapsid carnivore functional feeding groups.
The feeding functional subgroup jaw functional character (Supplementary Methods) distributions illustrated using violin and box plots. Functional feeding group compositions illustrated using ring plots detailing relative proportions of different taxonomic groups. Violin plots show taxon density. Box plots showing median value and upper and lower quartiles, with whisker illustrating standard deviation. Mean values indicate by black dots. Coloured arrows indicate whether values increase (red) or decrease (blue) relevant jaw functionality. N = 122. Jaw silhouettes (left to right): Varanodon agilis, Tetraceratops insignis, Dimetrodon grandis, Sauroctonus parringtoni, Smilesaurus ferox, Annatherapsidus petri, Tetracynodon darti. BIA Biarmosuchia, CYN Cynodontia, DIN Dinocephalia, EOT Eothyrididae, GRG Gorgonopsia, MAMA mean anterior mechanical advantage, MAR maximum aspect ratio, MPMA mean posterior mechanical advantage, OMA opening mechanical advantage, OPH Ophiacodontidae, RAO relative articulation offset, RSL relative symphyseal length, RTL relative toothrow length, SA Symphyseal angle, SPH Sphenacodontia (non-therapsid), THR Therocephalia, VAR Varanopidae. N = 122 taxa. Biarmosuchia, Dinocephalia and Therocephalia silhouettes by Dmitry Bogdanov (vectorized by T. Michael Keesey); all other silhouettes created by S.A.S., but some are vectorised from artwork by Felipe Alves Elias (https://www.paleozoobr.com/), available for academic use with attribution.Fig. 3: The ecofunctional focus of synapsid carnivore functional feeding groups.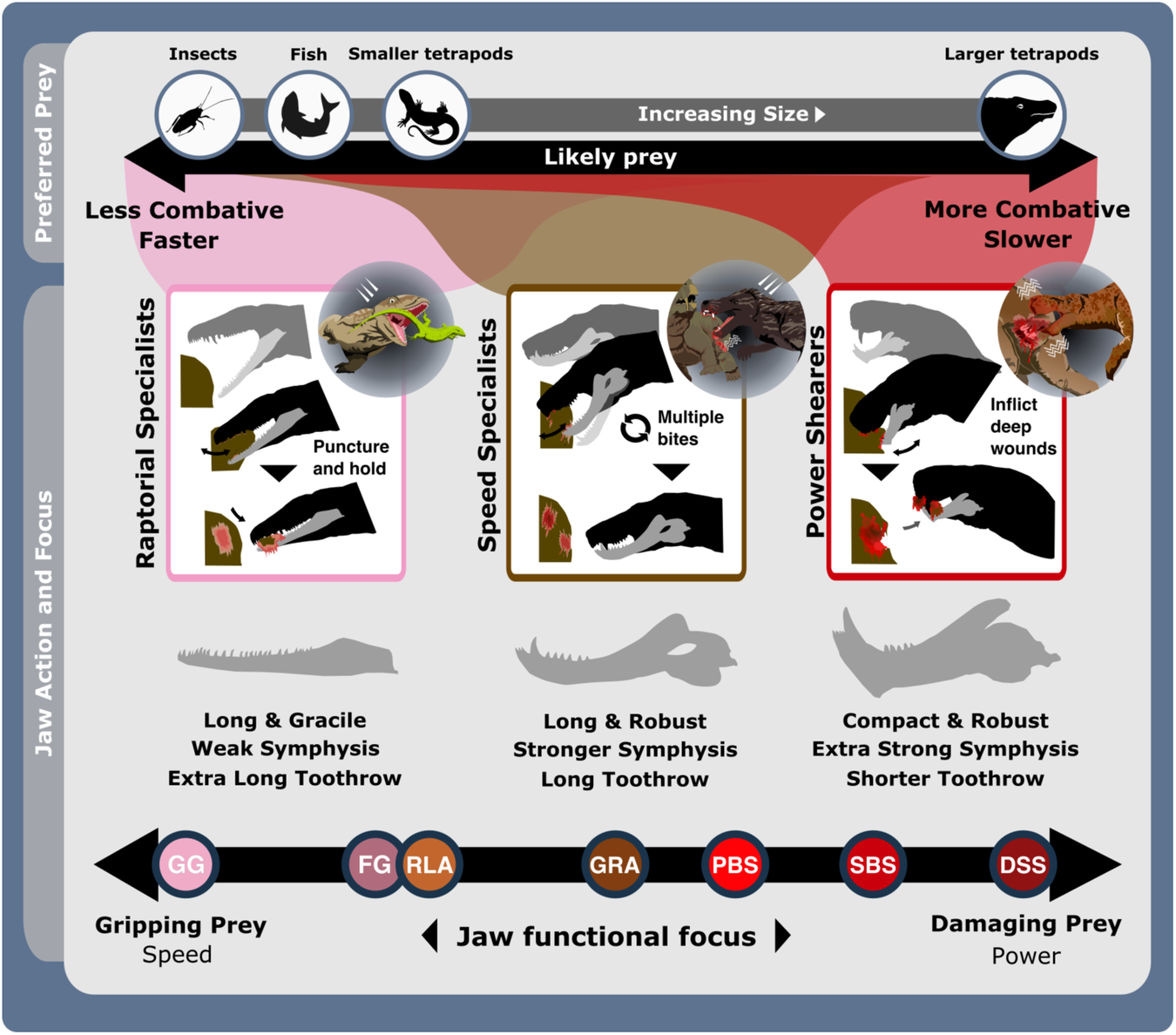 Likely prey preferences and capture methods of the raptorial specialist, speed specialist, power shearer functional feeding groups, as suggested by overall interpretation of jaw functional traits. Jaw silhouettes (left to right): Mesenosaurus romeri, Annatherapsidus petri, Aelurognathus tigriceps. DSS deep shearing specialist, FG forceful gripper, GG gracile gripper, GRA grip and rip attacker, PBS power bite specialist, RLA rapid light attacker, SBS shearing bite specialist, SS speed specialist. All silhouettes created by S.A.S.Fig. 4: Synapsid carnivore jaw morpho-functional evolution and relative abundance through time.
Likely prey preferences and capture methods of the raptorial specialist, speed specialist, power shearer functional feeding groups, as suggested by overall interpretation of jaw functional traits. Jaw silhouettes (left to right): Mesenosaurus romeri, Annatherapsidus petri, Aelurognathus tigriceps. DSS deep shearing specialist, FG forceful gripper, GG gracile gripper, GRA grip and rip attacker, PBS power bite specialist, RLA rapid light attacker, SBS shearing bite specialist, SS speed specialist. All silhouettes created by S.A.S.Fig. 4: Synapsid carnivore jaw morpho-functional evolution and relative abundance through time. a Jaw shape and functional morphospace changes through the late Palaeozoic. Morphospace margin colours correspond to colours of the relevant time bin on the stratigraphic chart. b Relative proportions of different taxonomic groups per time bin through the late Palaeozoic. ART Artinskian, ASL Asselian, BIA Biarmosuchia, CAP Capitanian, CHX Changhsingian, CRC Carboniferous rainforest collapse, CYN Cynodontia, DIN Dinocephalia, ECE End-Capitanian extinction, EOT Eothyrididae, GRG Gorgonopsia, GZH Gzhelian, IND Induan, KAS Kasimovian, KUN Kungurian, OE Olson’s extinction, OPH Ophiacodontidae, PENN Pennsylvanian, PTME Permo-Triassic mass extinction, SAK Sakmarian, SPH Sphenacodontia (non-therapsid), ROA Roadian, THR Therocephalia, VAR Varanopidae, WOR Wordian, WUC Wuchiapingian. Biarmosuchia, Dinocephalia and Therocephalia silhouettes by Dmitry Bogdanov (vectorized by T. Michael Keesey); all other silhouettes created by S.A.S., but some are vectorised from artwork by Felipe Alves Elias (https://www.paleozoobr.com/), available for academic use with attribution.Fig. 5: Synapsid carnivore jaw shape and functional phylogenetic disparity through the late Palaeozoic.
a Jaw shape and functional morphospace changes through the late Palaeozoic. Morphospace margin colours correspond to colours of the relevant time bin on the stratigraphic chart. b Relative proportions of different taxonomic groups per time bin through the late Palaeozoic. ART Artinskian, ASL Asselian, BIA Biarmosuchia, CAP Capitanian, CHX Changhsingian, CRC Carboniferous rainforest collapse, CYN Cynodontia, DIN Dinocephalia, ECE End-Capitanian extinction, EOT Eothyrididae, GRG Gorgonopsia, GZH Gzhelian, IND Induan, KAS Kasimovian, KUN Kungurian, OE Olson’s extinction, OPH Ophiacodontidae, PENN Pennsylvanian, PTME Permo-Triassic mass extinction, SAK Sakmarian, SPH Sphenacodontia (non-therapsid), ROA Roadian, THR Therocephalia, VAR Varanopidae, WOR Wordian, WUC Wuchiapingian. Biarmosuchia, Dinocephalia and Therocephalia silhouettes by Dmitry Bogdanov (vectorized by T. Michael Keesey); all other silhouettes created by S.A.S., but some are vectorised from artwork by Felipe Alves Elias (https://www.paleozoobr.com/), available for academic use with attribution.Fig. 5: Synapsid carnivore jaw shape and functional phylogenetic disparity through the late Palaeozoic.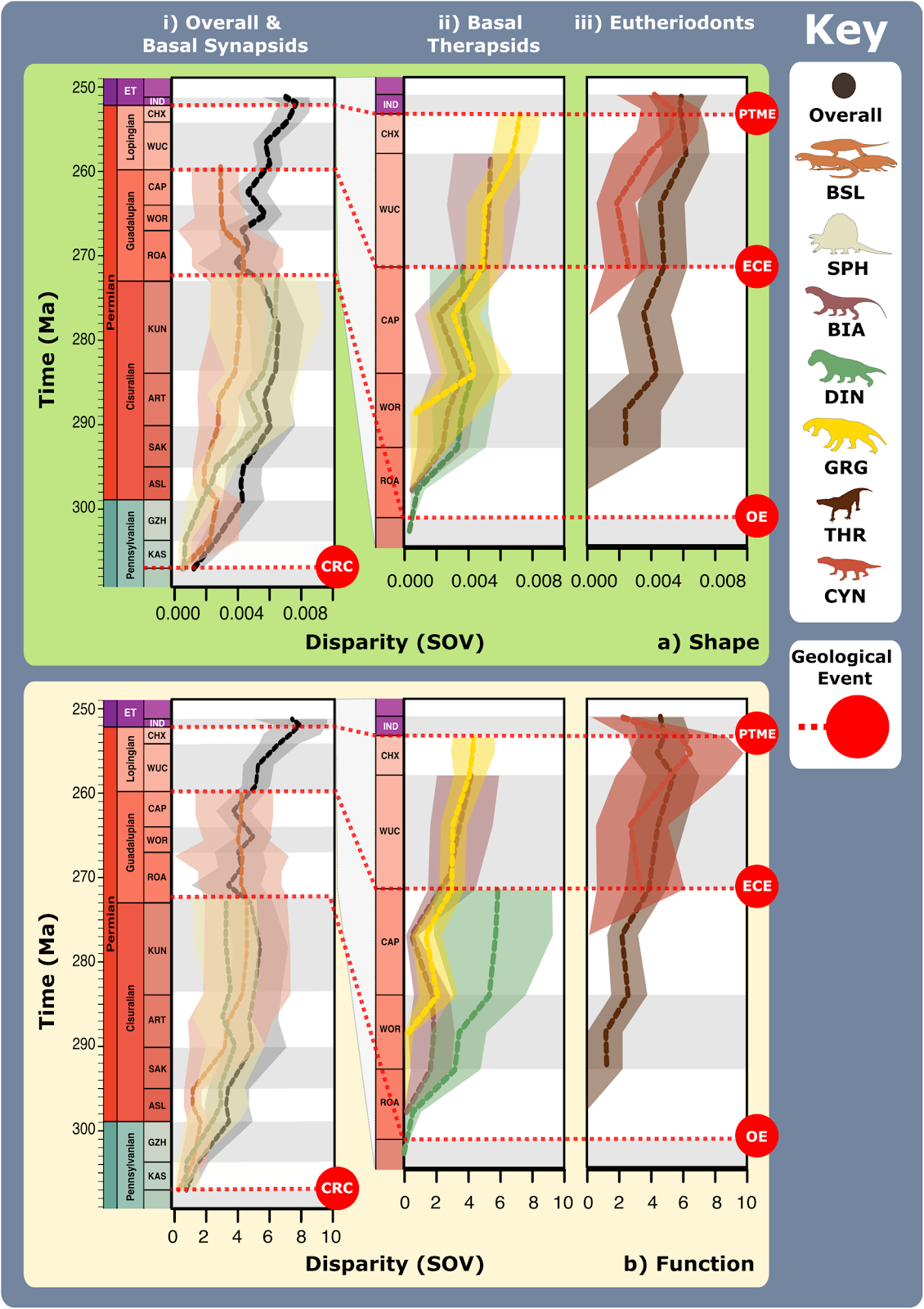 a Shape and (b) functional sum of variance calculated for each time bin for carnivorous synapsid groups using phylogenetic time-slicing70, divided into: (i) Basal synapsids, (ii) Basal therapsids, and (iii) Eutheriodonts. Significant geological events also highlighted. ‘Overall’ represents all carnivorous synapsids. Shaded 95% confidence intervals shown for each curve. N = 122. ART Artinskian, ASL Asselian, BIA Biarmosuchia, BSL Basal-most synapsids (eothyridids, varanopids, and ophiacodonts), CAP Capitanian, CHX Changhsingian, CRC Carboniferous rainforest collapse, CYN Cynodontia, DIN Dinocephalia, ECE End-Capitanian extinction, GRG Gorgonopsia, GZH Gzhelian, IND Induan, KAS Kasimovian, KUN Kungurian, OE Olson’s extinction, PENN Pennsylvanian, PTME Permo-Triassic mass extinction, SAK Sakmarian, SPH Sphenacodontia (non-therapsid), ROA Roadian, THR Therocephalia, WOR Wordian, WUC Wuchiapingian. Biarmosuchia, Dinocephalia and Therocephalia silhouettes by Dmitry Bogdanov (vectorized by T. Michael Keesey); all other silhouettes created by S.A.S., but some are vectorised from artwork by Felipe Alves Elias (https://www.paleozoobr.com/), available for academic use with attribution.Fig. 6: Synapsid carnivore feeding functional subgroups through the late Palaeozoic.
a Shape and (b) functional sum of variance calculated for each time bin for carnivorous synapsid groups using phylogenetic time-slicing70, divided into: (i) Basal synapsids, (ii) Basal therapsids, and (iii) Eutheriodonts. Significant geological events also highlighted. ‘Overall’ represents all carnivorous synapsids. Shaded 95% confidence intervals shown for each curve. N = 122. ART Artinskian, ASL Asselian, BIA Biarmosuchia, BSL Basal-most synapsids (eothyridids, varanopids, and ophiacodonts), CAP Capitanian, CHX Changhsingian, CRC Carboniferous rainforest collapse, CYN Cynodontia, DIN Dinocephalia, ECE End-Capitanian extinction, GRG Gorgonopsia, GZH Gzhelian, IND Induan, KAS Kasimovian, KUN Kungurian, OE Olson’s extinction, PENN Pennsylvanian, PTME Permo-Triassic mass extinction, SAK Sakmarian, SPH Sphenacodontia (non-therapsid), ROA Roadian, THR Therocephalia, WOR Wordian, WUC Wuchiapingian. Biarmosuchia, Dinocephalia and Therocephalia silhouettes by Dmitry Bogdanov (vectorized by T. Michael Keesey); all other silhouettes created by S.A.S., but some are vectorised from artwork by Felipe Alves Elias (https://www.paleozoobr.com/), available for academic use with attribution.Fig. 6: Synapsid carnivore feeding functional subgroups through the late Palaeozoic. a Relative abundance through time of different feeding functional (sub)groups. b Mean body sizes for each feeding functional subgroup through time. c Composition of each functional feeding group by functional subgroup and clade per time bin. Incorporates unsampled lineages using ancestral trait estimation of overall jaw shape and linear discriminant analysis for FFsG classification. Key geological events shown. Epochs are colour coded by period: Carboniferous (green), Permian (orange), and Triassic (purple). N = 122. ART Artinskian, ASL Asselian, BIA Biarmosuchia, CAP Capitanian, CHX Changhsingian, CYN Cynodontia, DIN Dinocephalia, DSS Deep shearing specialist, ECE End-Capitanian extinction, EOT Eothyrididae and assorted Casesauria, ET Early Triassic, FFsG feeding functional subgroup, FG forceful gripper, GG gracile gripper, GRA grip and rip attacker, GRG Gorgonopsia, GZH Gzhelian, IND Induan, KAS Kasimovian, KUN Kungurian, OE Olson’s extinction, OPH Ophiacodontidae, PBS Power bite specialist, Penn Pennsylvanian, PTME Permo-Triassic mass extinction, RLA Rapid light attacker, ROA Roadian, SAK Sakmarian, SBS Shearing bite specialist, SPH Sphenacodontia (non-therapsid), THR Therocephalia, VAR Varanopidae, WOR Wordian, WUC Wuchiapingian. Biarmosuchia, Dinocephalia and Therocephalia silhouettes by Dmitry Bogdanov (vectorized by T. Michael Keesey); all other silhouettes created by S.A.S., but some are vectorised from artwork by Felipe Alves Elias (https://www.paleozoobr.com/), available for academic use with attribution.Fig. 7: Synapsid carnivore ecomorphological evolution through the late Palaeozoic.
a Relative abundance through time of different feeding functional (sub)groups. b Mean body sizes for each feeding functional subgroup through time. c Composition of each functional feeding group by functional subgroup and clade per time bin. Incorporates unsampled lineages using ancestral trait estimation of overall jaw shape and linear discriminant analysis for FFsG classification. Key geological events shown. Epochs are colour coded by period: Carboniferous (green), Permian (orange), and Triassic (purple). N = 122. ART Artinskian, ASL Asselian, BIA Biarmosuchia, CAP Capitanian, CHX Changhsingian, CYN Cynodontia, DIN Dinocephalia, DSS Deep shearing specialist, ECE End-Capitanian extinction, EOT Eothyrididae and assorted Casesauria, ET Early Triassic, FFsG feeding functional subgroup, FG forceful gripper, GG gracile gripper, GRA grip and rip attacker, GRG Gorgonopsia, GZH Gzhelian, IND Induan, KAS Kasimovian, KUN Kungurian, OE Olson’s extinction, OPH Ophiacodontidae, PBS Power bite specialist, Penn Pennsylvanian, PTME Permo-Triassic mass extinction, RLA Rapid light attacker, ROA Roadian, SAK Sakmarian, SBS Shearing bite specialist, SPH Sphenacodontia (non-therapsid), THR Therocephalia, VAR Varanopidae, WOR Wordian, WUC Wuchiapingian. Biarmosuchia, Dinocephalia and Therocephalia silhouettes by Dmitry Bogdanov (vectorized by T. Michael Keesey); all other silhouettes created by S.A.S., but some are vectorised from artwork by Felipe Alves Elias (https://www.paleozoobr.com/), available for academic use with attribution.Fig. 7: Synapsid carnivore ecomorphological evolution through the late Palaeozoic. Feeding functional subgroup states cross the carnivorous synapsid phylogeny with reconstructed ancestral character state likelihoods based on mean recovered states under equal, symmetrical, asymmetrical, and all rates different models of character transition denoted by pie charts at node positions. Positions of key clades indicated by numbers in bold across the phylogeny. Pulses of diversification highlighted with shaded boxes (grey for carnivorous synapsids and pale green for tetrapod herbivores). Body size represented by Log10(mm) femur length, with branch colour denoting low or high values (see scale). Key geological events shown. N = 122. ART Artinskian, ASL Asselian, BIA Biarmosuchia, CAP Capitanian, CHX Changhsingian, CYN Cynodontia, DIN Dinocephalia, DSS Deep shearing specialist, ECE End-Capitanian extinction, EOT Eothyrididae, ET Early Triassic, FFsG feeding functional subgroup, FG forceful gripper, GG gracile gripper, GRA grip and rip attacker, GRG Gorgonopsia, GZH Gzhelian, I Induan, KAS Kasimovian, KUN Kungurian, MOS Moscovian, OE Olson’s extinction, OPH Ophiacodontidae, PBS Power bite specialist, PTME Permo-Triassic mass extinction, RLA Rapid light attacker, ROA Roadian, SAK Sakmarian, SBS Shearing bite specialist, SPH Sphenacodontia (non-therapsid), THR Therocephalia, VAR Varanopidae, WO Wordian, WUC Wuchiapingian. Biarmosuchia, Dinocephalia and Therocephalia silhouettes by Dmitry Bogdanov (vectorized by T. Michael Keesey); all other silhouettes created by S.A.S., but some are vectorised from artwork by Felipe Alves Elias (https://www.paleozoobr.com/), available for academic use with attribution.Fig. 8: Feeding functional subgroup and size differentiation within carnivorous synapsid assemblages through the late Palaeozoic.
Feeding functional subgroup states cross the carnivorous synapsid phylogeny with reconstructed ancestral character state likelihoods based on mean recovered states under equal, symmetrical, asymmetrical, and all rates different models of character transition denoted by pie charts at node positions. Positions of key clades indicated by numbers in bold across the phylogeny. Pulses of diversification highlighted with shaded boxes (grey for carnivorous synapsids and pale green for tetrapod herbivores). Body size represented by Log10(mm) femur length, with branch colour denoting low or high values (see scale). Key geological events shown. N = 122. ART Artinskian, ASL Asselian, BIA Biarmosuchia, CAP Capitanian, CHX Changhsingian, CYN Cynodontia, DIN Dinocephalia, DSS Deep shearing specialist, ECE End-Capitanian extinction, EOT Eothyrididae, ET Early Triassic, FFsG feeding functional subgroup, FG forceful gripper, GG gracile gripper, GRA grip and rip attacker, GRG Gorgonopsia, GZH Gzhelian, I Induan, KAS Kasimovian, KUN Kungurian, MOS Moscovian, OE Olson’s extinction, OPH Ophiacodontidae, PBS Power bite specialist, PTME Permo-Triassic mass extinction, RLA Rapid light attacker, ROA Roadian, SAK Sakmarian, SBS Shearing bite specialist, SPH Sphenacodontia (non-therapsid), THR Therocephalia, VAR Varanopidae, WO Wordian, WUC Wuchiapingian. Biarmosuchia, Dinocephalia and Therocephalia silhouettes by Dmitry Bogdanov (vectorized by T. Michael Keesey); all other silhouettes created by S.A.S., but some are vectorised from artwork by Felipe Alves Elias (https://www.paleozoobr.com/), available for academic use with attribution.Fig. 8: Feeding functional subgroup and size differentiation within carnivorous synapsid assemblages through the late Palaeozoic.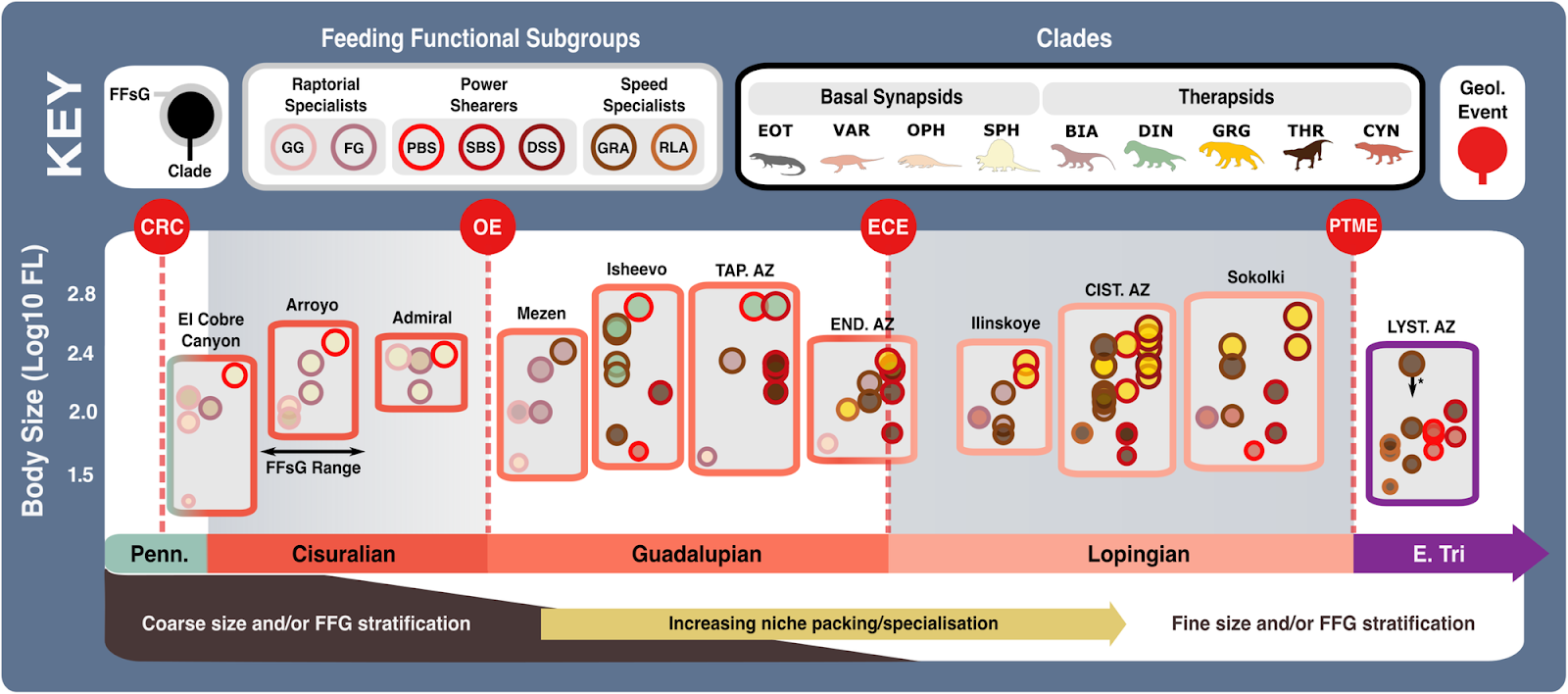 The feeding functional subgroup classifications and size of carnivorous synapsids within late Palaeozoic fossil assemblages, illustrating potential ecological similarity and changes in niche differentiation. Body size represented by Log10(mm) femur length. *Size based on Permian specimen as Early Triassic specimen with complete femur length measurement could not be sourced—Early Triassic specimens are typically smaller owing to Lilliput effect across the PTME73. Key geological events shown. Epochs are colour coded by period: Carboniferous (green), Permian (orange), and Triassic (purple). N = 81. BIA Biarmosuchia, CIST. AZ Cistecephalus Assemblage Zone149, CYN Cynodontia, DIN Dinocephalia, DSS Deep shearing specialist, ECE End-Capitanian extinction, END. AZ Endothiodon Assemblage Zone (Lycosuchus—Eunotosaurus subzone)148, EOT Eothyrididae, E. Tri Early Triassic, FFsG Feeding functional subgroup, FG Forceful gripper, FL Femur length, GG Gracile gripper, GRA Grip and rip attacker, GRG Gorgonopsia, Lcm Locomotion, LYST. AZ Lystrosaurus declivis Assemblage Zone89, OE Olson’s extinction, OPH Ophiacodontidae, PBS Power bite specialist, Penn Pennsylvanian, PTME Permo-Triassic mass extinction, RLA Rapid light attacker, SBS Shearing bite specialist, SPH Sphenacodontia (non-therapsid), TAP. AZ Tapinocephalus Assemblage Zone (Diictodon—Styracocephalus subzone)147, THR Therocephalia, VAR Varanopidae. Biarmosuchia, Dinocephalia and Therocephalia silhouettes by Dmitry Bogdanov (vectorized by T. Michael Keesey); all other silhouettes created by S.A.S., but some are vectorised from artwork by Felipe Alves Elias (https://www.paleozoobr.com/), available for academic use with attribution.
The feeding functional subgroup classifications and size of carnivorous synapsids within late Palaeozoic fossil assemblages, illustrating potential ecological similarity and changes in niche differentiation. Body size represented by Log10(mm) femur length. *Size based on Permian specimen as Early Triassic specimen with complete femur length measurement could not be sourced—Early Triassic specimens are typically smaller owing to Lilliput effect across the PTME73. Key geological events shown. Epochs are colour coded by period: Carboniferous (green), Permian (orange), and Triassic (purple). N = 81. BIA Biarmosuchia, CIST. AZ Cistecephalus Assemblage Zone149, CYN Cynodontia, DIN Dinocephalia, DSS Deep shearing specialist, ECE End-Capitanian extinction, END. AZ Endothiodon Assemblage Zone (Lycosuchus—Eunotosaurus subzone)148, EOT Eothyrididae, E. Tri Early Triassic, FFsG Feeding functional subgroup, FG Forceful gripper, FL Femur length, GG Gracile gripper, GRA Grip and rip attacker, GRG Gorgonopsia, Lcm Locomotion, LYST. AZ Lystrosaurus declivis Assemblage Zone89, OE Olson’s extinction, OPH Ophiacodontidae, PBS Power bite specialist, Penn Pennsylvanian, PTME Permo-Triassic mass extinction, RLA Rapid light attacker, SBS Shearing bite specialist, SPH Sphenacodontia (non-therapsid), TAP. AZ Tapinocephalus Assemblage Zone (Diictodon—Styracocephalus subzone)147, THR Therocephalia, VAR Varanopidae. Biarmosuchia, Dinocephalia and Therocephalia silhouettes by Dmitry Bogdanov (vectorized by T. Michael Keesey); all other silhouettes created by S.A.S., but some are vectorised from artwork by Felipe Alves Elias (https://www.paleozoobr.com/), available for academic use with attribution.
Singh, S.A., Elsler, A., Stubbs, T.L. et al.
Predatory synapsid ecomorphology signals growing dynamism of late Palaeozoic terrestrial ecosystems. Commun Biol 7, 201 (2024). https://doi.org/10.1038/s42003-024-05879-2
Copyright: © 2024 The authors.
Published by Springer Nature Ltd. Open access.
Reprinted under a Creative Commons Attribution 4.0 International license (CC BY 4.0)
Obviously (to anyone who understands evolution) while the synapsids were evolving to get better at catching and killing their prey as quickly as possible, their prey was also evolving to get better at avoiding being caught. Each species acted as an environmental selector in the other's environment
What Makes You So Special? From The Big Bang To You
Ten Reasons To Lose Faith: And Why You Are Better Off Without It




No comments:
Post a Comment
Obscene, threatening or obnoxious messages, preaching, abuse and spam will be removed, as will anything by known Internet trolls and stalkers, by known sock-puppet accounts and anything not connected with the post,
A claim made without evidence can be dismissed without evidence. Remember: your opinion is not an established fact unless corroborated.