Genetic basis for the evolution of hair discovered in the clawed frog
"When sorrows come, they come not single spies but in battalions" - Claudius in Hamlet.
That was never more true of creationism than it is today with the publication of not just the usual casual refutation of creationism we've come to expect most days, but of four disparate papers each of which casually and unintentionally refutes creationism to anyone who understands biology and is familiar with the basic dogmas of the creation cult, simply by revealing real-world facts.
The papers range from this one, which shows how mammalian hair has its genetic origins in a common human-amphibian ancestor, through how a female butterfly evolved Batesian mimicry, through the discovery of a giant Amazonian dolphin from 16 million years before creationists think Earth existed, to how early modern humans survived a super-volcano eruption in South-West Ethiopia a mere 64,000 years before 'Creation Week'.
With so many papers I'll do my best to cover all of them in the next few days, so keep checking back!
Firstly, the evolutionary origin of mammalian hair.
The gene for this originated in a common ancestor of humans (and the other mammals) and a modern clawed frog. The gene controls the growth of keratin, of which the claws of a clawed frog are composed, as is mammalian hair. The evidence for this common origin was found by researchers from the Medical University of Vienna, Austria, led by led by Leopold Eckhart. The team have published their findings open access in Nature Communications and described their work in a Medical University of Vienna new release:
(Vienna, 18 March 2024) The development of hair was of central importance for the evolution of mammals and thus also of humans. However, the evolutionary origin of the genetic programme of hair was previously unknown. An international research team led by Leopold Eckhart from MedUni Vienna has now been able to show that important hair components and their genetic control have already evolved in amphibians. Human hair therefore shows unexpected similarities to the claws of clawed frogs. The results were recently published in the scientific journal "Nature Communications".
In order to investigate the evolution of skin appendages, which include human hair and nails, the MedUni Vienna research team, in collaboration with the University of Ghent (Belgium), used the tropical clawed frog (Xenopus tropicalis) as an experimental model. The study revealed that the cornified claws of Xenopus frogs consist of special proteins (keratins) that are very similar to the main components of mammalian hair and nails. The formation of these keratins was found to be controlled by a specific gene, Hoxc13, in both humans and frogs.
It is known that patients with mutations in the Hoxc13 gene have defects in the growth of hair and nails. In our study, we were able to block the formation of claws in the clawed frog by switching off this gene," reports Leopold Eckhart from MedUni Vienna's Department of Dermatology. These results indicate that the genetic programme for the development of keratinized claws originated in a common ancestor of humans and frogs. "During the evolution of mammals, the programme of claw formation was modified for the development of hair," says Eckhart.
Important research question clarified
The evolution of terrestrial vertebrates is characterized by the appearance of an effective skin barrier against water loss in a dry environment and by the development of hard, keratinized skin appendages such as claws, scales, feathers and hair, which are crucial for catching prey, protection, supporting special types of locomotion and thermal insulation. The evolution of skin appendages is therefore an important research question. The findings from the project, which is funded by the Austrian Science Fund (FWF), contribute to clarifying the evolutionary origin of keratinized skin appendages and also help to better understand the regulation of hair in humans. "Our publication will stimulate further exciting studies in basic and preclinical research," concludes Leopold Eckhart.
AbstractThe fact that both amphibians and mammals have versions of the Hoxc13 gene which carry out related functions to do with keratin production is of course evidence of common origins. It is also evidence of evolution by genetic modification, in other words, by mutation and selection and how evolution will use pre-existing genes for novel purposes, in this case for controlling the growth of hair in mammals - something that was never the original function of the Hoxc13 gene. Hoxc13 has been modified during evolution as the original amphibian skin became modified for a fully terrestrial existence as the tetrapods left the water completely and evolved into first the reptiles, then the mammals, carrying the Hoxc13 gene with them.
Cornified skin appendages, such as hair and nails, are major evolutionary innovations of terrestrial vertebrates. Human hair and nails consist largely of special intermediate filament proteins, known as hair keratins, which are expressed under the control of the transcription factor Hoxc13. Here, we show that the cornified claws of Xenopus frogs contain homologs of hair keratins and the genes encoding these keratins are flanked by promoters in which binding sites of Hoxc13 are conserved. Furthermore, these keratins and Hoxc13 are co-expressed in the claw-forming epithelium of frog toe tips. Upon deletion of hoxc13, the expression of hair keratin homologs is abolished and the development of cornified claws is abrogated in X. tropicalis. These results indicate that Hoxc13-dependent expression of hair keratin homologs evolved already in stem tetrapods, presumably as a mechanism for protecting toe tips, and that this ancestral genetic program was coopted to the growth of hair in mammals.
Introduction
The evolution of terrestrial vertebrates is characterized by the appearance of an efficient barrier against water loss in a dry environment and by the evolution of hard cornified skin appendages, such as claws, scales, feathers, and hair, which are critical for the capture of prey, protection, support of special modes of locomotion, and thermoinsulation1,2,3. Cornified skin appendages are characteristic features of important phylogenetic clades of terrestrial vertebrates, exemplified by hair in mammals and feathers in birds. Thus, the evolution of skin appendages is a major research question in the biology of tetrapods2.
Cornified skin appendages consist of terminally differentiated and inter-connected keratinocytes which are packed with specific cytoskeletal and other structural proteins. The keratinocytes within the mature parts of hard skin appendages such as hair, feathers, claws and nails are dead, meaning that they lack gene expression and metabolism. Importantly, the aforementioned skin appendages are regenerated either continuously in the case of cornified claws and nails or cyclically in the case of hair and feathers. Thus, the program of keratinocyte differentiation and cornification is not only active during embryonic development but also in the adult organism. Mammalian cornified skin appendages are characterized by the expression of so-called hair keratins, i.e. keratin intermediate filament proteins which were originally identified in hair4. Human hair keratins are comprised of KRT31-KRT40, which are type I keratins, and KRT81-KRT86, which are type II keratins5. Type I and type II hair keratins heterodimerize to form intermediate filaments. Not only hair, but also claws, nails, horns, filiform papillae of the tongue, and other hard skin appendages of mammals contain hair keratins5. We identified homologs of hair keratins in the green anole lizard and showed that these keratins are expressed in the cornified claws6, which are homologous to human nails. The conserved expression in claws and nails suggested that (1) proteins of the type I and II “hair keratin” families originated prior to the divergence of mammals and reptiles, and (2) the primordial sites of “hair keratin expression” were not hair follicles but other structures that were likely homologous to claws in extant reptiles and mammals. Further studies led to the identification of hair keratin-like proteins, though distinguished from human hair keratins by a low content of cysteine, in amphibians7,8,9. These homologs of hair keratins are expressed in toe pads of tree frogs10, but it has remained unknown where they are expressed in phylogenetically diverse amphibians with claw-like structures, such as clawed frogs of the genus Xenopus, clawed salamanders of the genus Onychodactylus and some sirens such as Pseudobranchus striatus11. Because of developmental and morphological differences to claws of amniotes, such as the accumulation of parallel cornified cell layers in claws of Xenopus frogs as opposed to the proximal-to-distal growth of human and mouse nails and claws of lizards, it was proposed that claws of clawed frogs had evolved independently from claws of amniotes11,12. However, a conclusive molecular analysis of amphibian claws has not been reported yet.
While structural proteins as components of cornified keratinocytes determine the hardness of skin appendages, the spatial and temporal pattern of expression of the corresponding genes and thereby the development and continuous regeneration of hard skin appendages are regulated at the level of gene transcription. One of the best characterized regulators of mammalian hair keratins is the transcription factor Hoxc13, which is expressed in the hair matrix and nail matrix13,14. Hoxc13 induces the expression of several hair keratin genes by binding to specific sites in the proximal promoters of hair keratin genes14. Mutations in human HOXC13 cause ectodermal dysplasia 9, a severe defect of hair and nails15,16. Likewise, mutations of Hoxc13 in mice, rabbits and pigs suppressed the growth of hair and nails17,18,19. The primordial function of Hox genes is to specify regions of the body along the head-tail axis during embryonic development20,21,22, whereby Hoxc13 as the terminal gene of the HoxC gene cluster is activated in the posterior part of the body, such as the tail fin of Actinopterygii (ray-finned fishes)23. During the evolution of Sarcopterygii (lobe-finned fishes), Hoxc13 was coopted to expression in paired fins, the evolutionary precursors of limbs in tetrapods24. Recent research has identified enhancer elements critical for the expression of HoxC genes in developing nails and hair follicles of mammals25. Importantly, the evolutionary steps between the role of Hoxc13 in fins of early Sarcopterygians and the regulatory role of Hoxc13 in mammalian hair and nails have remained elusive.
Here, we investigated the evolutionary origin of the link between Hoxc13 and hair keratins. We show that amphibian homologs of hair keratins are components of claws in the Western clawed frog (X. tropicalis), a model for basal tetrapods. The expression of these keratins and the formation of cornified claws were abrogated by the targeted disruption of hoxc13 in X. tropicalis, which is equivalent to the absence of hair and nails in mammals upon genetic inactivation of Hoxc13. Our results highlight a conserved role of Hoxc13 in claws and hair and link the evolution of limbs with the evolution of cornified skin appendages in tetrapods.
Fig. 1: Synteny analysis and molecular phylogenetics identify hair keratin homologs in amphibians. a, b Schematic representation of the keratin type I (a) and type II (b) gene clusters of selected vertebrates: human, Homo sapiens; frog, Xenopus tropicalis; axolotl, Ambystoma mexicanum; caecilian, Rhinatrema bivittatum; lungfish, Protopterus annectens. Genes are shown as triangles pointing in the direction of transcription. Homologs of hair keratin genes are highlighted by blue shading. Note that KRT18 is the only type I keratin gene located in the type II cluster. Slanted double lines indicate sites where genes are omitted for clarity. c, d Phylogenetic analysis of type I (c) and type II (d) keratins. Keratin orthologs in amphibians and human or lungfish with bootstrap values > 50 (indicated in c, d) are shown with matching colors in panels (a) and (c) (type I) and (b) and (d) (type II). Nodes without numbers have bootstrap values < 50. Note the strong support (bootstrap >90) for orthology of amphibian krt34 (c) and krt59 (d) with human hair keratins (highlighted in blue) of the respective keratin type. Only fully sequenced keratin genes were included in this analysis. e Model for the evolution of hair keratin homologs in relation to the evolution of amphibians54. mya, million years ago; +, present; -, absent.
a, b Schematic representation of the keratin type I (a) and type II (b) gene clusters of selected vertebrates: human, Homo sapiens; frog, Xenopus tropicalis; axolotl, Ambystoma mexicanum; caecilian, Rhinatrema bivittatum; lungfish, Protopterus annectens. Genes are shown as triangles pointing in the direction of transcription. Homologs of hair keratin genes are highlighted by blue shading. Note that KRT18 is the only type I keratin gene located in the type II cluster. Slanted double lines indicate sites where genes are omitted for clarity. c, d Phylogenetic analysis of type I (c) and type II (d) keratins. Keratin orthologs in amphibians and human or lungfish with bootstrap values > 50 (indicated in c, d) are shown with matching colors in panels (a) and (c) (type I) and (b) and (d) (type II). Nodes without numbers have bootstrap values < 50. Note the strong support (bootstrap >90) for orthology of amphibian krt34 (c) and krt59 (d) with human hair keratins (highlighted in blue) of the respective keratin type. Only fully sequenced keratin genes were included in this analysis. e Model for the evolution of hair keratin homologs in relation to the evolution of amphibians54. mya, million years ago; +, present; -, absent.
Fig. 6: Model for the evolutionary origin of Hoxc13 and hair keratin-dependent cornified skin appendages.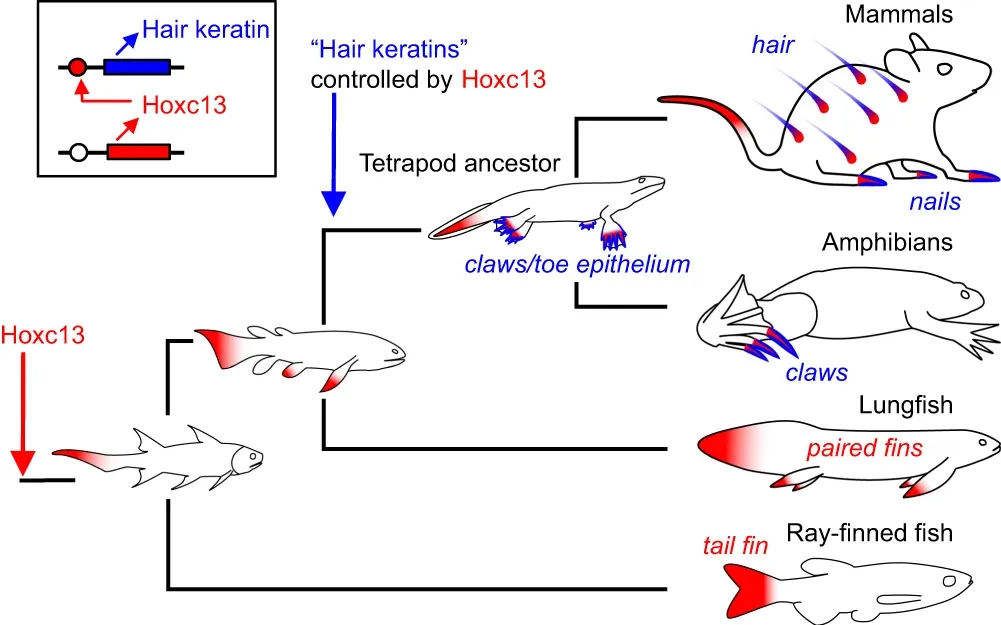 The evolutionary changes in the expression pattern of Hoxc13 and the appearance of the regulatory link between Hoxc13 and “hair keratins” were inferred from data obtained in extant vertebrates and their known phylogeny. “Hair keratins” refers to human keratins KRT31-KRT40 and KRT81-KRT86 and their orthologs in other species. Red and blue shading mark sites of expression of Hoxc13 and hair keratin homologs, respectively.
The evolutionary changes in the expression pattern of Hoxc13 and the appearance of the regulatory link between Hoxc13 and “hair keratins” were inferred from data obtained in extant vertebrates and their known phylogeny. “Hair keratins” refers to human keratins KRT31-KRT40 and KRT81-KRT86 and their orthologs in other species. Red and blue shading mark sites of expression of Hoxc13 and hair keratin homologs, respectively.
Carron, M., Sachslehner, A.P., Cicekdal, M.B. et al.
Evolutionary origin of Hoxc13-dependent skin appendages in amphibians. Nat Commun 15, 2328 (2024). https://doi.org/10.1038/s41467-024-46373-x
Copyright: © 2025 The authors.
Published by Springer Nature Ltd. Open access.
Reprinted under a Creative Commons Attribution 4.0 International license (CC BY 4.0)
Those creationists who can be relied on to trot out the traditional excuse for evidence of common origins - that they are evidence of a common designer - need to explain why their putative designer either gave a frog a gene for growing mammalian hair or gave mammals a gene for growing frog claws. Or perhaps it just forged the genetic evidence to make it look like humans and frogs have a common ancestor in order to make us believe it doesn't exist and a natural process is responsible for the present-day forms of living organisms.
ID is not a problem for science; rather science is a problem for ID. This book shows why. It exposes the fallacy of Intelligent Design by showing that, when examined in detail, biological systems are anything but intelligently designed. They show no signs of a plan and are quite ludicrously complex for whatever can be described as a purpose. The Intelligent Design movement relies on almost total ignorance of biological science and seemingly limitless credulity in its target marks. Its only real appeal appears to be to those who find science too difficult or too much trouble to learn yet want their opinions to be regarded as at least as important as those of scientists and experts in their fields.
Available in Hardcover, Paperback or ebook for Kindle
This book explains why faith is a fallacy and serves no useful purpose other than providing an excuse for pretending to know things that are unknown. It also explains how losing faith liberates former sufferers from fear, delusion and the control of others, freeing them to see the world in a different light, to recognise the injustices that religions cause and to accept people for who they are, not which group they happened to be born in. A society based on atheist, Humanist principles would be a less divided, more inclusive, more peaceful society and one more appreciative of the one opportunity that life gives us to enjoy and wonder at the world we live in.
Available in Hardcover, Paperback or ebook for Kindle





No comments:
Post a Comment
Obscene, threatening or obnoxious messages, preaching, abuse and spam will be removed, as will anything by known Internet trolls and stalkers, by known sock-puppet accounts and anything not connected with the post,
A claim made without evidence can be dismissed without evidence. Remember: your opinion is not an established fact unless corroborated.