What makes a pathogen antibiotic-resistant? | Sanford Burnham Prebys
One of todays examples of the stupidity of creationism is something of a novelty. Usually, by applying the central tenets of creationism, any putative designer of living things like parasites either appears malevolent (and sometime it has to be said, malevolent at a near genius level in the ways it finds to make us and other animals sick) or it looks incompetent in that its 'solutions' are often to problems of its own making and more often than not to solutions it designed for one side of an arms race which it now trets as problems for the other side.
But today's example can only be described as an example of incompetent malevolennce, as creationism's putative designer, faced with the same 'problem' of medical science developing antibiotics effective against two different species of pathogen, set about designign two completely different 'solutions' to this problem. - Talk about re-inventing the wheel!
The pathogens are: Escherichia coli and Acinetobacter baumannii.
Creationists will probably be familiar with Escherichia coli (E. coli) because they believe their guru, Michael J Behe, 'proved' their god exists by claiming (falsely) that E. coli's flagellum must have been intelligently designed because he didn't know it evolved out of a pre-exiting structure and couldn't think how else it could have evolved. But then such is the standard of creationist apologetics!
What Behe had unwitting done was destroy the traditional excuse creationists use to explain pathogens like E. coli by blaming them on another 'designer' called 'Sin' which somehow creates living organisms although the creationit designer god is the only entitiy capable of designing livign things, so any example of 'intelligent design, real or imaginary, if 'proof' of this designer god's existance.
So, what creationists are now left with is an E.coli with a flegellum designed by their god to make it better at making us sick, and now resitant to antibiotics to help it win against medical science trying to prevent it makign us sick!
But what creationists are less likely to be familiar with is the pathogen, Acinetobacter baumannii, so here is a little background:
What information do you have on the pathogen, Acinetobacter baumannii? Acinetobacter baumannii is a Gram-negative bacterium that has gained significant attention due to its ability to cause infections, particularly in healthcare settings. Here's some information about this pathogen:How the discovery was made that these two pathogens have developed antibiotic resistance in two entirely different ways is the subjec of an open access paper in npj Antimicrobials and Resistance and a news release from the research insitute Sanford Burnham Prebys where the lead rearcher, Dr. Andrei L. Osterman, is based:Overall, Acinetobacter baumannii poses a significant challenge in healthcare settings due to its ability to cause infections, its resistance to multiple antibiotics, and its persistence in the environment. Efforts to prevent and control its spread are essential for minimizing the impact of infections caused by this pathogen.
- Classification: Acinetobacter baumannii is a member of the genus Acinetobacter, which includes several other species. It is a non-motile, aerobic bacterium.
- Healthcare-Associated Infections (HAIs): Acinetobacter baumannii is known for causing various healthcare-associated infections, including pneumonia, bloodstream infections, urinary tract infections, and wound infections. These infections often occur in patients who are already ill or have compromised immune systems, such as those in intensive care units (ICUs).
- Antibiotic Resistance: One of the most concerning aspects of Acinetobacter baumannii is its ability to develop resistance to multiple antibiotics. It has become notorious for its resistance to many commonly used antibiotics, including carbapenems, which are often considered the last line of defense against multidrug-resistant bacteria. This resistance can make infections difficult to treat and can lead to increased morbidity and mortality rates.
- Environmental Resilience: Acinetobacter baumannii is known for its ability to survive in various environments, including hospital surfaces and medical equipment. This resilience contributes to its persistence in healthcare settings and its potential to cause outbreaks.
- Virulence Factors: Acinetobacter baumannii possesses various virulence factors that contribute to its ability to cause infections. These include outer membrane proteins, lipopolysaccharides, and enzymes that aid in evasion of the host immune response and colonization of host tissues.
- Prevention and Control: Due to its resistance and ability to cause healthcare-associated infections, preventing the spread of Acinetobacter baumannii is crucial. This involves strict infection control measures in healthcare settings, including hand hygiene, environmental cleaning, and appropriate use of antibiotics to minimize the development of resistance.
In a new study, Sanford Burnham Prebys researchers, led by Andrei L. Osterman, Ph.D., combined experimental evolution in...
Posted by Sanford Burnham Prebys on Thursday, 7 March 2024
Researchers compared two common bacterial foes and two specific drugs, looking for deeper explanations and clinical implications.Technical details are given in the team's open access paper in npj Antimicrobials and Resistance:
Antimicrobial resistance is a story of constantly moving parts and players. With every new or tweaked antibiotic or antimicrobial drug, the targeted pathogens begin the evolutionary dance of acquiring resistance, prompting researchers to constantly develop workarounds or entirely new classes of medicine.
Understanding the underlying mechanisms of acquired antimicrobial resistance is critical to the fight, a case of knowing one’s enemy. In a new paper published March 2, 2024 in npj Antimicrobials and Resistance, part of the Nature Portfolio, researchers at Sanford Burnham Prebys, working with Roche Pharma Research and Early Development, describe how two notable pathogens—Escherichia coli and Acinetobacter baumannii—employ distinctly different tools to fend off antibiotic attack.
Both pathogens studied are gram-negative bacteria with shared characteristics, but also notable differences.This work was conceived as a comparative study of the mechanisms and dynamics of resistance acquisition for two drugs and two bugs. Comparing mutational landscapes triggered by the same drug in two distinct bugs allows us to deduce both shared and unique evolutionary trajectories toward resistance. A comparison of two drugs in the same bug reveals shared and unique mechanistic features of fundamental and translational importance, from drug discovery to rational optimization of treatment regimens.
Dr. Andrei L. Osterman, Ph.D., senior author
Sanford Burnham Prebys.
For the most part, E. coli is a bacterium routinely found in the guts of humans and animals, where it resides with no ill effect. Some strains, however, do cause harm, everything from mild gastroenterological distress to urinary tract infections, respiratory illness and pneumonia.
Acinetobacter baumannii is more problematic, particularly in clinical settings where it can cause severe infections, some life-threatening.
Andei Osterman, Ph.D.Both bacteria have developed resistance to most current antibiotic treatments.
In their paper, Osterman and colleagues combined experimental evolution in a continuous culturing device (morbidostat) with whole genome sequencing of evolving cultures to track how E. coli and A. baumannii acquired drug resistance against a pair of antibiotics that inhibit DNA gyrase, an essential enzyme in bacteria.
Inhibiting the enzyme disrupts DNA synthesis and, subsequently, causes the bacteria to die. One antibiotic—ciprofloxacin—has been in active clinical use since 1987; the other—GP6—is experimental.
The researchers found that pathogenic bacteria with acquired resistance to ciprofloxacin remained susceptible to effective antimicrobial treatment by GP6 drug. However, the opposite was not true: evolution of resistance to GP6 also triggered resistance to ciprofloxacin.
These findings underscored that E. coli and A. baumannii employ shared and unique mechanisms to acquire resistance to these two types of drugs.
Bacteria acquire resistance as a result of random mutational events that happen in the DNA replication as uncorrected ‘typos. These spontaneously emerge in a handful of drug-resistant variants/strains out of godzillions of neutral mutations) under selective pressure.
Dr. Andrei L. Osterman, Ph.D
(A godzillion is a descriptive term, not a precise unit of measurement. It refers to a number of enormous size. In this case a number ranging between 108 to 109 neutral mutations.)
These harmful mutations may underlie several types of resistance mechanisms, including modifying the protein targets of a given drug, the ability of bacterial cells to expel compounds (drugs) before they cause harm (efflux) and special bacterial enzymes that inactivate active drug compounds.
Osterman said the work advances progress toward developing “resistibility profiles” of established and novel antimicrobial drugs, which would help set forth “boundaries for possible combinational treatment, including clinically relevant multidrug resistant strains. Our acquired knowledge provides crucial guidelines for all these translational activities.”
The findings are not limited to E. coli and A. baumannii. They have potential to at least partially predict resistance drivers across other closely related species posing significant health risks, including Pseudomonas, Salmonella and Klebsiella spp. all difficult-to-treat bacterial pathogens linked to serious and sometimes deadly infections.
Additional authors on the study include Semen A. Leyn, James E. Kent, Jaime E. Zlamal and Marinela L. Elane, all at Sanford Burnham Prebys; and Maarten Vercruysse, Rocha Pharma.
AbstractRegular readers of these blog posts and of my illustrated book, The Malevolent Designer: Why Nature's God is not Good, will be more that a little acquainted with the idea that, if there were such a ting as an intelligent designer, it can only be regarded as malevolent in that is puts so much effort into designing ways to make its creation suffer with parasites such as viruses, bacteria, worms of all descriptions and protozoa such as Plasmodium falciparum and Trypanosoma brucei that cause malaria and sleepign sickness respectively.
Comprehensive knowledge of mechanisms driving the acquisition of antimicrobial resistance is essential for the development of new drugs with minimized resistibility. To gain this knowledge, we combine experimental evolution in a continuous culturing device, the morbidostat, with whole genome sequencing of evolving cultures followed by characterization of drug-resistant isolates. Here, this approach was used to assess evolutionary dynamics of resistance acquisition against DNA gyrase/topoisomerase TriBE inhibitor GP6 in Escherichia coli and Acinetobacter baumannii. The evolution of GP6 resistance in both species was driven by a combination of two classes of mutational events: (i) amino acid substitutions near the ATP-binding site of the GyrB subunit of the DNA gyrase target; and (ii) various mutations and genomic rearrangements leading to upregulation of efflux pumps, species-specific (AcrAB/TolC in E. coli and AdeIJK in A. baumannii) and shared by both species (MdtK). A comparison with the experimental evolution of resistance to ciprofloxacin (CIP), previously performed using the same workflow and strains, revealed fundamental differences between these two distinct classes of compounds. Most notable were non-overlapping spectra of target mutations and distinct evolutionary trajectories that, in the case of GP6, were dominated by upregulation of efflux machinery prior to (or even in lieu) of target modification. Most of the efflux-driven GP6-resistant isolates of both species displayed a robust cross-resistance to CIP, while CIP-resistant clones showed no appreciable increase in GP6-resistance.
Introduction
DNA gyrase GyrA/B and topoisomerase IV ParC/E are bacterial type II topoisomerases. Both enzyme complexes are vital for DNA replication, transcription and decatenation of daughter chromosomes in bacteria and have significant structural and sequence differences from the human type II topoisomerases1. These proteins are established targets for common clinical antibiotics of the fluoroquinolone class2 and for new drug discovery campaigns3,4.
The mechanism of action of fluoroquinolones involves binding at the topoisomerase-DNA interface, which allows DNA cleavage while suppressing the ligation reaction, thus introducing multiple deleterious nicks in the chromosome5. Despite excellent efficacy against a broad spectrum of bacterial pathogens, the clinical utility of fluoroquinolones is hampered by rapidly spreading resistance driven largely by amino acid substitutions in a drug-binding site of its cognate targets that do not impair their enzymatic functions6. These limitations of fluoroquinolones prompted numerous efforts to develop new type II topoisomerase inhibitors with a different binding mode and mechanism of action to avoid cross-resistance7. Thus, Novobiocin, an aminocoumarin class antibiotic targeting the ATP-binding site of GyrB, was introduced into clinical practice in the 1960s, but discontinued in 1999 due to insufficient efficacy and safety8. Two non-quinolone antibiotics have reached Phase III of clinical trials: (i) Zoliflodacin, a first-in-class spiropyrimidinetrione antibiotic9; and (ii) Gepotidacin, which belongs to a novel class of triazaacenaphthylenes10. These drugs bind in different sites at the topoisomerase-DNA interface showing no cross-resistance with fluoroquinolones. A novel tricyclic class of pyrimidoindole GyrB/ParE inhibitors (TriBE inhibitors) active against a broad range of Gram-negative bacterial pathogens was introduced by Trius Therapeutics in 201311.
The goal of this study was to assess the mutational landscape and evolutionary dynamics of resistance acquisition against the TriBE inhibitor GP612 in the model system of Escherichia coli and in the important nosocomial pathogen Acinetobacter baumannii. This knowledge is essential for fundamental mechanistic understanding and for a comparative assessment of bacterial resistibility to this novel drug candidate. To this end, we applied a workflow combining experimental evolution in a continuous culturing device, the morbidostat, with high-coverage whole genome sequencing (WGS) of evolving bacterial populations followed by genotype-to-phenotype characterization of selected drug-resistant clones. This workflow (introduced in a model study of triclosan13 and schematically illustrated in Supplementary Fig. S1) was recently applied to the experimental evolution of resistance of three Gram-negative bacterial species to the most common fluoroquinolone drug ciprofloxacin (CIP)14. This study revealed shared evolutionary trajectories toward CIP resistance driven by a limited set of missense mutations in the GyrA/B target (Stage I) followed by additional mutations leading to upregulation of species-specific efflux pumps (Stage II). In A. baumannii, (but not in E. coli), additional Stage II mutational events included amino acid substitutions in the secondary target ParC/E that also led to a substantial increase of the MIC of CIP (MICCIP). This work provided a technological and conceptual foundation for a comparative resistomics assessment of a non-quinolone GyrB/ParE inhibitor GP6 reported here.
The performed analysis revealed fundamental mechanistic differences between these two distinct classes of type II topoisomerase inhibitors. Observed differences encompass non-overlapping spectra of target mutations and distinct evolutionary trajectories, dominated by deregulation of the efflux machinery in Stage I of evolution of resistance to GP6, prior to or even in lieu of target mutations. These differences also manifest in asymmetric cross-resistance profiles. While selected GP6 resistant (GP6R) clones displayed a comparably increased resistance to CIP, none of the previously characterized CIP resistant (CIPR) clones showed any increase in the MIC of GP6 (MICGP6). Overall, this study illustrates the utility of the morbidostat-based comparative resistomics approach for the assessment of resistibility of new drug candidates.Fig. 1: Cumulative area plots reflecting the evolutionary dynamics of the two major types of driver mutations. (i) target modifications (red area) and (ii) mutations leading to efflux upregulation (blue area); and other potentially relevant mutations (grey area). In a simplified form, the plots represent WGS data obtained for population samples in time series of collected during experimental evolution of resistance to GP6 in E. coli and A. baumannii in this study vs CIP from the previous study14. The variant abundance data corresponding to each of the 6 reactors (5 in case of A. baumannnii_CIP) were summed up by the three categories and plotted as the average number of mutations per cell (Y-axis) vs time (X-axis). The plots reflect combined relative abundances of SNV and IS insertions, but not CNVs (loci amplifications or deletions) that cannot be accurately quantified in population WGS data (reflected in separate plots in Supplementary Fig. S3).Fig. 2: Three-dimensional projection of detected mutations in DNA gyrase complex conferring resistance to CIP or GP6 drugs.
(i) target modifications (red area) and (ii) mutations leading to efflux upregulation (blue area); and other potentially relevant mutations (grey area). In a simplified form, the plots represent WGS data obtained for population samples in time series of collected during experimental evolution of resistance to GP6 in E. coli and A. baumannii in this study vs CIP from the previous study14. The variant abundance data corresponding to each of the 6 reactors (5 in case of A. baumannnii_CIP) were summed up by the three categories and plotted as the average number of mutations per cell (Y-axis) vs time (X-axis). The plots reflect combined relative abundances of SNV and IS insertions, but not CNVs (loci amplifications or deletions) that cannot be accurately quantified in population WGS data (reflected in separate plots in Supplementary Fig. S3).Fig. 2: Three-dimensional projection of detected mutations in DNA gyrase complex conferring resistance to CIP or GP6 drugs. a The structure of E. coli DNA Gyrase complex with DNA (PDB ID: 6RKW). GyrA subunits are colored shades of green; GyrB subunits are colored shades of blue; DNA is colored yellow. The location of GP6 (orange, modeled from 4KSG) and CIP (pink, modeled from PDB ID: 5BTC) binding sites are depicted by spheres. The amino acid residues (colored magenta) detected as mutational variants during the morbidostat-based evolution of resistance to GP6 or CIP are also shown. The areas outlined by two dashed boxes are expanded to focus on (b) GP6 and (c) CIP (variants present in the second molecule of the homodimer are not labeled).
a The structure of E. coli DNA Gyrase complex with DNA (PDB ID: 6RKW). GyrA subunits are colored shades of green; GyrB subunits are colored shades of blue; DNA is colored yellow. The location of GP6 (orange, modeled from 4KSG) and CIP (pink, modeled from PDB ID: 5BTC) binding sites are depicted by spheres. The amino acid residues (colored magenta) detected as mutational variants during the morbidostat-based evolution of resistance to GP6 or CIP are also shown. The areas outlined by two dashed boxes are expanded to focus on (b) GP6 and (c) CIP (variants present in the second molecule of the homodimer are not labeled).
Fig. 3: Mutational events in efflux systems observed in experimental evolution of GP6 resistance in E. coli and A. baumannii; comparison with CIP resistance studies14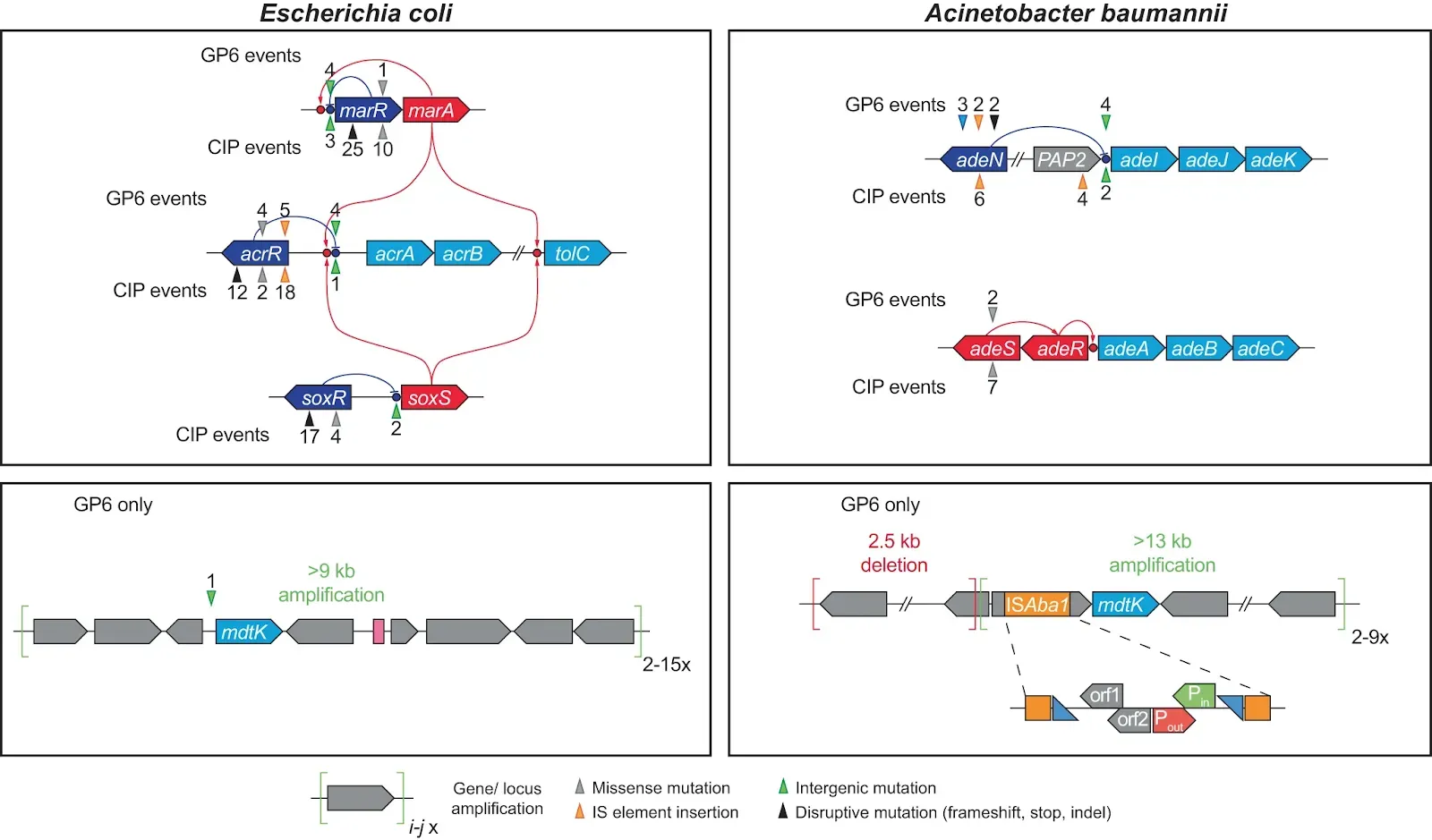 Gene coloring scheme: light blue – components of efflux transporters; dark blue – transcriptional repressors; red – transcriptional activators (including two-component systems); Grey – other genes. GP6-selected mutational events are shown above the affected genes, CIP-selected – below the genes. The positions of mutational events are shown by triangles (colored depending on type of events as shown below the diagram) and numbers reflecting the number of their independent occurrences.
Gene coloring scheme: light blue – components of efflux transporters; dark blue – transcriptional repressors; red – transcriptional activators (including two-component systems); Grey – other genes. GP6-selected mutational events are shown above the affected genes, CIP-selected – below the genes. The positions of mutational events are shown by triangles (colored depending on type of events as shown below the diagram) and numbers reflecting the number of their independent occurrences.
Fig. 4: The order of occurrence and impact of mutations and their combinations driving the evolution of resistance to GP6 in E. coli and A. baumannii, a comparison with the experimental evolution of CIP resistance (modified from ref. 14). Only main driver mutations represented in characterized clones (from Supplementary Table S3A–D) are shown leading to: (i) target modification (orange background palette); and (ii) efflux upregulation (blue background palette). The impact of these mutations on MIC (fold change) of respective drugs (MICGP6 or MICCIP) is shown above the corresponding variants. Intermediate variants (mostly, single mutants not represented in isolated clones) hypothesized based on dynamic plots (Supplementary Fig. S3) are indicated by dashed borders and arrows.
Only main driver mutations represented in characterized clones (from Supplementary Table S3A–D) are shown leading to: (i) target modification (orange background palette); and (ii) efflux upregulation (blue background palette). The impact of these mutations on MIC (fold change) of respective drugs (MICGP6 or MICCIP) is shown above the corresponding variants. Intermediate variants (mostly, single mutants not represented in isolated clones) hypothesized based on dynamic plots (Supplementary Fig. S3) are indicated by dashed borders and arrows.
Fig. 5: Correlation plot of changes in MIC values of GP6 and CIP compounds in E. coli and A. baumannii. MIC values were observed over a panel of ~40 nonredundant clones selected from morbidostat-based studies on experimental evolution of resistance to GP6 (yellow and green symbols) or CIP (orange and light-brown symbols) in E. coli (triangles) and A. baumannii (circles). Corresponding mutational variants (only driver mutations) are shown as data labels using a matching font color. Complete mutational and MIC data are provided in Supplementary Table S3A–D. Plotted values reflect fold-change of MIC (FC) measured for both drugs in each clone as compared to a respective MIC of the unevolved parental strain. Two outlined areas illustrate a clear separation between CIPR-evolved isolates (that do not show any increase in MIC to GP6) and GP6 R-evolved isolates (many of which show an appreciable cross-resistance to CIP).
MIC values were observed over a panel of ~40 nonredundant clones selected from morbidostat-based studies on experimental evolution of resistance to GP6 (yellow and green symbols) or CIP (orange and light-brown symbols) in E. coli (triangles) and A. baumannii (circles). Corresponding mutational variants (only driver mutations) are shown as data labels using a matching font color. Complete mutational and MIC data are provided in Supplementary Table S3A–D. Plotted values reflect fold-change of MIC (FC) measured for both drugs in each clone as compared to a respective MIC of the unevolved parental strain. Two outlined areas illustrate a clear separation between CIPR-evolved isolates (that do not show any increase in MIC to GP6) and GP6 R-evolved isolates (many of which show an appreciable cross-resistance to CIP).
Leyn, S.A., Kent, J.E., Zlamal, J.E. et al.
Two classes of DNA gyrase inhibitors elicit distinct evolutionary trajectories toward resistance in gram-negative pathogens. npj Antimicrob Resist 2, 5 (2024). https://doi.org/10.1038/s44259-024-00021-y
Copyright: © 2024 The authors.
Published by Springer Nature. Open access
Reprinted under a Creative Commons Attribution 4.0 International license (CC BY 4.0)
But I think this is the first case I've come across where, any putative intelligent designer would have to be so fanatically devoted to making us sick that it has deigned two different ways for pathogenic bacteria to win in the latest twist in the arms race between them and human medical science in the form of antibiotic resistance.
The fact that creationists would prefer us to think of their putative designer in such a light, or to take from it the 'unique' ability to create livign organiams and give that to 'Sin' as well, shows us that the agenda of the creation cult is not to promulgate worship and wonder of what they claim is an all-lovign god, but to win recruits for as sect with a hidden, political agenda. Creationism was perhaps the first of the extreme right, post-truth cults who argue that you can't trust the experts so should believe what those ignorant of the facts tell you - an alternative view of reality that says ignore the evidence and believe what I tell you to believe.
And the creationist dupes willingly queue up to be fooled by them because they've bought into the notion that their childish, fairy-tale fantasy world is a better description of it than anything science can provide, because their ignorant intuition trumps facts.
The Malevolent Designer: Why Nature's God is Not Good
Illustrated by Catherine Webber-Hounslow.
The Unintelligent Designer: Refuting The Intelligent Design Hoax






No comments:
Post a Comment
Obscene, threatening or obnoxious messages, preaching, abuse and spam will be removed, as will anything by known Internet trolls and stalkers, by known sock-puppet accounts and anything not connected with the post,
A claim made without evidence can be dismissed without evidence. Remember: your opinion is not an established fact unless corroborated.