New mathematical model sheds light on the absence of breastfeeding in male mammals - News and events, University of York
Ask an intelligent [sic] design creationists why males have nipples, and you'll get lots of evasion and avoidance but no answer beyond mutterings about an 'ineffable god', and 'not knowing the minds of the creator', betraying the religious underpinning of creationism.
So, why exactly do males have nipples?
The answer is, of course, evolution.
Why do male mammals have nipples? Male mammals have nipples because they develop early in the embryonic stage, before the differentiation of sex characteristics. Here's a more detailed explanation:Firstly, the genes which control the development of nipples in the embryo are not on the sex chromosomes so males have whatever females need; secondly, the development of the breast tissues into milk-producing mammary glands is controlled by hormones produced by ovaries, so, although males have the genetic potential for lactation and breast-feeding, the appropriate glands never develop - except, that is, in one mammal - the Dayak fruit bat from the Malaysian forests, both sexes of which breastfeed their young.
In summary, male mammals have nipples because they are formed early in the embryonic development process, before the body distinguishes itself as male or female. This trait is retained throughout evolution due to the lack of negative impact on survival or reproduction.
- Embryonic Development: In mammals, all embryos initially follow a similar developmental pathway. During the early stages of development, embryos are essentially unisex, having the potential to develop into either males or females. Nipples form during this period, before the sex of the embryo is determined.
- Genetic and Hormonal Factors: The differentiation into male or female happens later in the developmental process, driven by the presence or absence of certain hormones and genetic factors. For males, the presence of the Y chromosome triggers the development of testes, which then produce testosterone. This hormone drives the development of male sex characteristics. However, by the time this differentiation occurs, the nipples have already formed.
- Evolutionary Perspective: From an evolutionary standpoint, there is no strong selection pressure to eliminate nipples in males because they do not cause harm or significant disadvantage. Thus, they are retained as a neutral trait.
- Mammalian Heritage: All mammals share a common ancestor, and this ancestor likely had a similar developmental process where nipples formed before sex differentiation. This trait has been passed down through evolutionary history.
Does this imply that an ancestral mammal male could have lactated?
The fact that male mammals have nipples due to early embryonic development does not necessarily imply that ancestral male mammals could lactate. However, it does open up interesting discussions about the evolutionary and biological potential for lactation in males. Here's a more detailed look:
In conclusion, while the anatomical potential for lactation exists in male mammals due to their embryonic development, it does not imply that ancestral male mammals routinely lactated. The development of efficient lactation mechanisms primarily evolved in females due to the direct reproductive advantage it provided.
- Basic Lactation Mechanism: Lactation is primarily driven by hormonal changes, particularly the hormones prolactin and oxytocin, which stimulate milk production and release. In females, these hormones surge during and after pregnancy, triggering the development of mammary glands and milk production.
- Potential for Male Lactation: While male mammals typically do not experience the hormonal changes necessary for lactation, the basic anatomical structures (including nipples and, in some cases, rudimentary mammary glands) are present. There have been rare instances where male mammals, including humans, have produced milk due to specific hormonal imbalances or external stimulation of the mammary glands.
- Evolutionary Context: The presence of nipples in male mammals does not necessarily mean that ancestral males lactated. It is more likely that the shared embryonic pathway leading to nipple development persisted because it posed no significant disadvantage. Over evolutionary time, the primary selection pressure was on females to develop efficient lactation mechanisms for nurturing offspring.
- Rare Phenomena: In certain species and under specific conditions, male lactation has been observed. For example, some male fruit bats have been documented to produce milk. These cases are exceptions rather than the rule and are usually attributed to unique evolutionary pressures or hormonal factors.
The reason for the near universal absence of lactation in male mammals, despite the obvious advantage that both parents being able to feed the offspring would seem to convey, was assumed to be due to the uncertainty of paternity, giving the increased probability that a male could be breastfeeding another male's offspring, contrasting with the certainty of maternity where there is no doubt that it is the female's genes that are benefitting from her breastfeeding. However, it's difficult to see how this would outweigh any advantages from both sexes breastfeeding
' But now three mathematicians from the University of York have developed a mathematic model which suggests the evolution of this difference in role and function could be due to something more powerful in terms of environmental drivers - it could be due to the increased risk of spreading harmful infections through the microbes that are found in breast milk. Their findings are the subject of an open access paper in the journal, Nature Communications and a news release from the University of York, UK:
New mathematical model sheds light on the absence of breastfeeding in male mammalsThe abstract and introduction to the paper in Nature Communications give the background to the paper which quickly become highly mathematical:
Being nursed by a single parent could be an evolutionary strategy to curb the spread of harmful microbes in mammals, according to a novel theory developed by mathematicians.
 The best dads in the primate world? Owl monkey fathers take on the role of primary caregiver and only hand their babies back to their female partners for nursing.
The best dads in the primate world? Owl monkey fathers take on the role of primary caregiver and only hand their babies back to their female partners for nursing.
The dense rainforest canopies of Malaysia are home to the only known case of a wild male mammal that produces milk. The Dayak fruit bat is a vanishingly rare case of male milk production, despite the fact that the potential for breastfeeding remains in place in most male mammals.
In the 1970s, evolutionary theorists posited that the near absence of lactation in males, even though offspring could benefit from the extra nutrition provided, could be attributed to the uncertainty of paternity: As male mammals can’t be sure they are the biological father, this reduces their evolutionary drive to invest heavily in offspring care, including breastfeeding.
Rich community
Now, mathematicians from the University of York have suggested a complementary perspective. Their hypothesis, published in Nature Communications, suggests that the reason male mammals don’t breastfeed might be driven by the rich community of microbes that lives in breast milk and which plays an important part in establishing the gut microbiome of the infant.
The theory demonstrates how the transmission of the milk microbiome from both parents would allow harmful microbes to spread through mammalian populations. Maternal-only lactation stops this as restricting transmission of the milk microbiome to females in effect acts as a sieve, retaining just the microbes with beneficial effects.
Devoted dads
We became fascinated with this topic when we read about Azara's owl monkeys. They turn previous assumptions about why males don’t breastfeed upside down because they are the most devoted dads in the primate world: They do 80–90% of childcare and only hand their babies back to their female partners for nursing.
When both parents are involved in feeding, the chance of a microbe being passed along and getting an initial foothold in a population is essentially doubled. So our theory suggests selection against the transmission of harmful microbes through mammary milk could be an additional selection pressure against male lactation.
Dr George W. A. Constable, co-author
Department of Mathematics
University of York, York, UK.
Complex ecosystem
Breast milk is a living substance and it plays a key role in establishing the gut microbiome of mammals, which is a complex ecosystem of bacteria, viruses and fungi, along with their genetic material. This ecosystem plays a crucial role in health including by helping to protect animals against disease, helping to digest food and in many other ways we are only just discovering.
While microbes are not inherently harmful or beneficial; it’s their presence and abundance that dictate the overall health of this internal community. A “wrong actor” at the early point of an animal’s life could change the microbiome at a pivotal moment.
Dr Brennen Fagan, first author
Leverhulme Centre for Anthropocene Biodiversity
And the Mathematics Department
University of York, York, UK.
Inevitable transmission
The mathematical model highlights the advantage of getting fed by just one parent, but the researchers say it makes evolutionary sense for this to be the mother because there has already been an inevitable transmission of microbes during birth and perhaps also in the womb.
This theory fits with a pattern of strategies mammals have adopted in an evolutionary bid to limit the spread of potentially harmful elements. Notably, in humans mitochondrial DNA is exclusively passed down from the mother. This mechanism serves as a natural filter, maintaining genetic integrity by suppressing the proliferation of detrimental mutations. Additionally, the prevalence of monogamous relationships among certain species has been suggested as an adaptive response aimed at minimising the transmission of sexually transmitted infections (STIs).
Dr George W. A. Constable.
Individual choices
The researchers caution that their hypothesis is not intended as the basis for any judgements about the different ways of feeding human infants.
Our model is very much focused on the long-term evolution of the animal kingdom. The model does not tell us about individual families making individual choices on how to safely feed their children, especially not for humans in the modern world. Our hypothesis fills a gap in evolutionary theory and is concerned with selection pressures on mammals at population level and over very long periods of time spanning multiple generations.
Dr Brennen Fagan.
AbstractThe principle relies on the fact that mammalian gut biota, which is normally regarded as symbiotic is actually a continuum from parasite to mutualist. Maternal only transmission acts as a barrier to the invasion of new detrimental 'symbionts' by reducing the transmission to half that if both parents were breastfeeding the young, but this could only have applied during evolution if maternal-only transmission applied. If both parents could transmit equally, then the advantage to the offspring in additional nutrients would outweigh any additional transmission of detrimental 'symbionts'. However, once there was an additional factor involved, reducing the paternal transmission, the female sieve would begin to operate reducing the advantage of paternal feeding further. That additional factor could have been the increased reproductive advantage to males of being free to mate with additional females.
Gut microbiomes of mammals carry a complex symbiotic assemblage of microorganisms. Feeding newborn infants’ milk from the mammary gland allows vertical transmission of the parental milk microbiome to the offspring’s gut microbiome. This has benefits, but also has hazards for the host population. Using mathematical models, we demonstrate that biparental vertical transmission enables deleterious microbial elements to invade host populations. In contrast, uniparental vertical transmission acts as a sieve, preventing these invasions. Moreover, we show that deleterious symbionts generate selection on host modifier genes that keep uniparental transmission in place. Since microbial transmission occurs during birth in placental mammals, subsequent transmission of the milk microbiome needs to be maternal to avoid the spread of deleterious elements. This paper therefore argues that viviparity and the hazards from biparental transmission of the milk microbiome, together generate selection against male lactation in placental mammals.
Introduction
The absence of male lactation in mammals is a puzzle—there appears to be no universally convincing reason why it should not happen. John Maynard Smith pointed out that paternal care that incorporates such feeding would be evolutionarily stable in monogamous mammals1. There have been over 200 My for male lactation to evolve2. Genetic control of the mammary gland is widely distributed across mammalian chromosomes3. Genetically male mammals have mammary tissue. They are known to have the potential to lactate4,5,6,7,8, and milk production has been recorded under natural conditions in the Dayak fruit bat (Dyacopterus spadiceus)4. Lactation requires hormonal triggers to take place, such as high levels of prolactin, which are generally down-regulated in males, preventing this from happening [e.g. ref. 9] It seems there are selection pressures preventing male lactation.
A well-known answer to the puzzle, building on the work of Trivers10,11, is that the absence of male lactation is simply a result of selection for sex-biased parental care. Should paternity be uncertain12,13, competition for female mates comparatively low14, or sexual selection on males high15, an evolutionary pressure exists for males to abandon care for their young in favour of additional mating opportunities. In such situations (in which male parental care is selected against), it is clear that the evolution of male lactation should be likewise prevented. However, should a combination of the above conditions not hold (i.e. paternity is more certain, competition for mates high, or sexual selection low), biparental care can instead evolve15. Indeed, this is the situation in around 10% of mammals16, amongst which Azara’s owl monkeys (Aotus azarae) are perhaps the starkest example; here the male is almost certain to be the father and the male is responsible for almost all care except for nursing17,18. This leads to the question of why, given other forms of paternal care have evolved19,20, male lactation remains the rare exception rather than the rule in these socially monogamous mammals.
This paper draws attention to a further set of participants in mammalian lactation which have important effects on the role of males. These are the microorganisms that form the milk microbiome, and that are transmitted from parent to offspring during feeding21. We call the association ‘symbiotic’ because this term is widely used to describe intimate and prolonged physical associations between dissimilar organisms—here a mammalian host and a microbial symbiont—irrespective of where the association lies on the mutualism-parasitism continuum22,23. We note that the term symbiotic is sometimes used as shorthand for mutualistic symbiotic interactions in the microbial literature [ref. 24, but see ref. 25], but we need the more general usage here, because this paper is concerned with how variation in the interactions drives natural selection on the host’s vertical transmission of microbes. These microbial symbionts could provide an evolutionary explanation for the absence of lactation in male mammals even when males invest in other forms of parental care.
Vertical transmission of symbionts through lactation, during the period from birth to weaning, provides a reliable conduit for moving microbes from host parent into the gut of host offspring. This is in contrast to horizontal transmission where symbionts are taken up from the environment in a less targeted way (see25 for a review of symbiont transmission). We focus on mammalian milk here, which contains its own microbiome, including bacteria, fungi and viruses21. Although just one of a number of channels for transmitting microbes from mammalian parents into the gut of their infants, milk is thought to make a major contribution to the infant’s gut microbiome early in life26, with ~103–104 colony-forming units of bacteria ml−1 in the case of human milk27. Vertical transmission of elements of the microbiome is well documented28; it is known to work across multiple host generations in mice29, and down to the level of specific clusters of parental strains of bacteria in the case of human milk30,31.
However, there is a basic, general danger to the host population from biparental transmission of symbionts. This is seen in its strongest form when a rare symbiont first colonises a host population, i.e. when most matings by hosts carrying the symbiont are with uninfected hosts (Fig. 1). When transmission is biparental, the symbiont is transmitted if it is carried by either host parent. In contrast, when transmission is uniparental (usually maternal), the symbiont has only one transmission route. Biparental transmission gives the symbiont a twofold reproductive boost when it is rare, enabling it to invade a host population, even if it is harmful to the host. (We note that harmful is a relative measure here, comparing the fitness of the host that carries the symbiont to the fitness of a host that does not.) Uniparental transmission removes this boost. This was recognised in early work on the evolution of uniparental cytoplasmic inheritance32,33, and was extended to the mixing of symbiotic lineages34, but not to vertical transmission of the gut microbiome. We develop our argument in the context of male lactation in mammals, but note that it may apply much more widely because mother-to-infant is thought to be the usual channel for symbiont transmission in animals with sexual reproduction35.
Here we use mathematical models to show that maternal transmission operates as a sieve on vertically transmitted symbionts. This sieve prevents invasion by a class of symbionts with deleterious effects on their hosts that would otherwise spread under biparental transmission. At the same time, the sieve still permits spread of symbionts with beneficial effects on their hosts. We demonstrate that deleterious symbionts also generate a selective advantage for host modifier genes preventing vertical transmission of symbionts through male hosts, and show that this selective advantage is maintained in the presence of some horizontal transmission of the deleterious symbionts. We note the significance of these results to the absence of male lactation in mammalian hosts: transmission of the milk microbiome to the gut microbiome of offspring is almost invariably from the mother. Although biparental milk production could bring nutritional benefits to offspring, we show that these benefits can be outweighed by the costs associated with deleterious symbionts.Fig. 1: Biparental transmission of symbionts in a host population gives the symbionts a reproductive boost.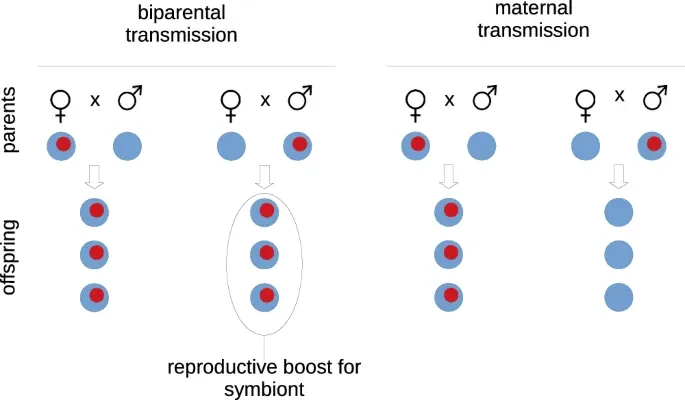 This is at its greatest when symbionts (in red) are rare in the host population, and infected hosts mate mostly with uninfected ones, as shown here. The boost is prevented by uniparental transmission, assumed here to be maternal.
This is at its greatest when symbionts (in red) are rare in the host population, and infected hosts mate mostly with uninfected ones, as shown here. The boost is prevented by uniparental transmission, assumed here to be maternal.
The mathematical modelling is complex but worth studying, since it shows how evolution can be represented by mathematical models which explain how environmental selectors operate to produce sometime counter-intuitive results - such as male nipped which serve no practical function but for which there are only very weak drivers to lose them altogether.
The idea of an intelligent designer giving males useless nipples is of course as absurd as is the idea that an intelligent, omniscient designer would grow nipples on an embryo before its sex was determined, even though its genetics were known, and regardless of whether they were going to have a function or not. But no doubt such absurdity impresses creationists no end, on the subjective grounds that a man without nipples would just look wrong, so their purpose is to make men look like men should look.



No comments:
Post a Comment
Obscene, threatening or obnoxious messages, preaching, abuse and spam will be removed, as will anything by known Internet trolls and stalkers, by known sock-puppet accounts and anything not connected with the post,
A claim made without evidence can be dismissed without evidence. Remember: your opinion is not an established fact unless corroborated.