Study identifies areas of Europe at risk from dengue fever | UK Centre for Ecology & Hydrology
For devotees of creationism's putative intelligent [sic] designer, news that it is using a new, improved mosquito to deliver dengue fever to more people, including those in the densely populated continent of Europe, will be greeted with admiration for its creative genius.
Those with a more rational, adult understanding of the evidence will see this news as a natural consequence of environmental change and exactly the sort of thing evolution can produce, precisely as the Theory of Evolution predicts.
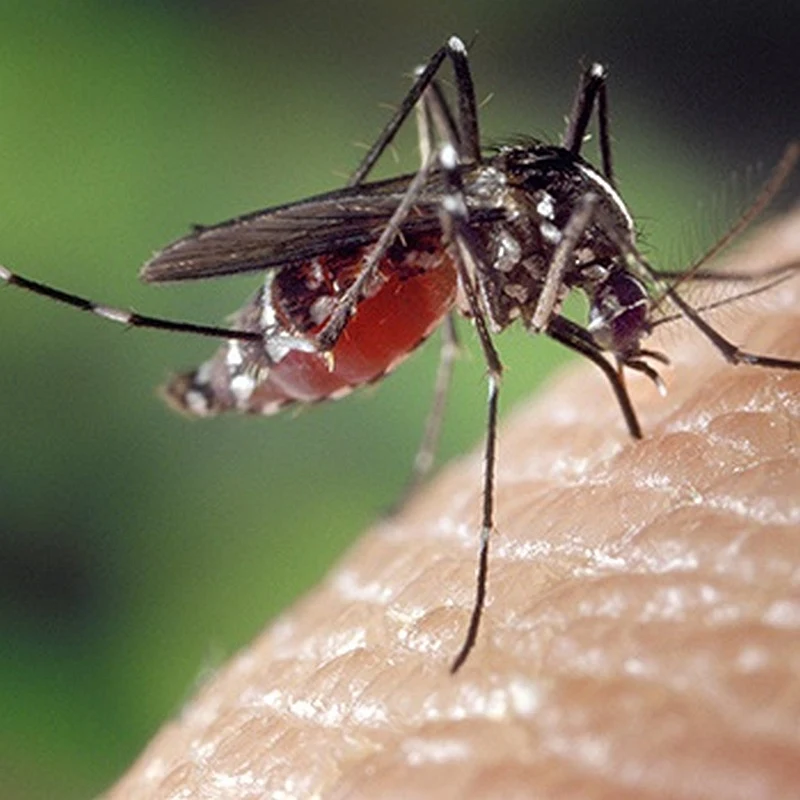
The news is that climate change has enabled the Asian tiger mosquito, Aedes albopictus, to extend its range into Europe and with it the virus that causes dengue fever or which the tiger mosquito is a vector.
Tell me all about the invasive Asian tiger mosquito, Aedes albopictus, and the threat it poses to human health. The Asian tiger mosquito (Aedes albopicus) is an invasive species native to Southeast Asia that has spread to many parts of the world, including North and South America, Europe, Africa, and the Middle East. It is a small, dark mosquito with distinctive white stripes on its legs and a white line running down the center of its back, giving it its "tiger" appearance.The results of a study lead by Dr Dominic Brass of the UK Centre of Ecology & Hydrology (UKCEH) have just been published, open access, in the journal Nature Communications, showing that it is possible to predict towns across Europe where there is a high risk of dengue fever.
Characteristics and Habitat
- Appearance: The Asian tiger mosquito is about 2-10 mm in length, black with white scales on its body and legs, and a characteristic white stripe running from the center of its head down its back.
- Behavior: Unlike many mosquito species that are active during dusk and dawn, Aedes albopicus is an aggressive daytime biter, particularly active during early morning and late afternoon.
- Breeding Habits: This mosquito breeds in small, water-filled containers, both natural (like tree holes and leaf axils) and artificial (like flowerpots, birdbaths, and discarded tires). It is well-adapted to urban and suburban environments.
- Distribution: The species has expanded its range globally due to its ability to withstand cooler temperatures and adapt to various environments. Its eggs can survive desiccation (drying out) for several months, which aids in its spread.
Health Threats
The Asian tiger mosquito poses significant public health risks because it is a vector for several viruses that cause diseases in humans:
- Dengue Fever: Aedes albopicus can transmit dengue viruses, which cause a severe flu-like illness characterized by high fever, severe headaches, pain behind the eyes, joint and muscle pain, rash, and mild bleeding. In severe cases, it can progress to dengue hemorrhagic fever or dengue shock syndrome, both of which can be fatal.
- Chikungunya: This mosquito is a primary vector for chikungunya, a viral disease that causes fever, severe joint pain, muscle pain, headache, nausea, fatigue, and rash. The joint pain can be debilitating and may last for weeks or even years.
- Zika Virus: Aedes albopicus is also capable of transmitting the Zika virus, which, while often causing mild symptoms like fever, rash, and joint pain, has been linked to serious complications such as microcephaly in babies born to infected mothers, as well as Guillain-Barré syndrome in adults.
- Yellow Fever: While the primary vector for yellow fever is the Aedes aegypti mosquito, Aedes albopicus can also transmit the virus. Yellow fever can cause fever, chills, severe headache, back pain, general body aches, nausea, vomiting, and fatigue. In severe cases, it can lead to liver damage, jaundice, and death.
- Other Viruses: The mosquito can also transmit other arboviruses, including West Nile virus, Japanese encephalitis, and certain filarial nematodes, though it is a less efficient vector for these diseases compared to other mosquito species.
Impact on Public Health and Economy
- Increased Disease Outbreaks: The spread of Aedes albopicus has been associated with outbreaks of dengue, chikungunya, and Zika virus in various regions. This poses a significant challenge to public health systems, especially in areas where these diseases were not previously endemic.
- Economic Burden: The presence of Aedes albopicus can result in significant economic costs related to healthcare, disease control, and prevention efforts. This includes expenses for mosquito control programs, public health campaigns, and hospitalization costs for infected individuals.
- Human Behavior Changes: The aggressive daytime biting behavior of this mosquito can affect people's quality of life, limiting outdoor activities and increasing the use of repellents and protective clothing.
Control Measures
- Source Reduction: Eliminating standing water where mosquitoes lay eggs is the most effective way to control Aedes albopicus. This includes cleaning and covering containers, removing debris, and managing natural water-holding sites.
- Biological Control: Natural predators like fish, certain bacteria (such as Bacillus thuringiensis israelensis), and fungi are used in some areas to control mosquito populations.
- Chemical Control: Insecticides (larvicides and adulticides) are used to reduce mosquito populations, but resistance and environmental concerns limit their use.
- Personal Protection: Using insect repellent, wearing protective clothing, and using mosquito nets or screens can help reduce exposure to mosquito bites.
- Genetic Control: Recent advances in genetic modification aim to reduce mosquito populations by releasing sterile or genetically altered mosquitoes that reduce the ability of the population to reproduce.
Conclusion
The Asian tiger mosquito poses a significant threat to human health due to its ability to transmit various viruses. As it continues to expand its range, effective control and public health measures are necessary to mitigate its impact and prevent outbreaks of mosquito-borne diseases.
They have concluded that it is possible to predict the effects of 'phenotypic plasticity' in A. albopicus, i.e., the evolutionary response to changes in the environment, particularly increased temperatures, which enable it to extend its range.
As the UKCEH news release explains:
Study identifies areas of Europe at risk from dengue fever
- Disease is spreading due to climate change, with outbreaks in France, Italy and Spain
- Surveillance for Asian tiger mosquitoes at Olympics highlights threat from invasive species
As Europe grapples with the growing threat of tropical diseases brought by the Asian tiger mosquito, a research breakthrough led by the UK Centre for Ecology & Hydrology (UKCEH) is enabling scientists to accurately predict towns across the continent where there is a high risk of dengue fever.
Global movement of people, trade and climate change, are now putting half the world’s population are at risk of contracting dengue fever*. Commonly referred to as the ‘bone breaker’, the disease may cause severe muscle and joint pain, and in some cases can result in internal bleeding and death.
The spread of the Asian tiger mosquito (Aedes albopictus) has caused local outbreaks of dengue in France including Paris, as well as Italy and Spain, contracting the virus by biting returning travellers who were infected while abroad and then transmitting it to other people in the area.
Scientists expect this invasive mosquito species to eventually gain a foothold in the UK.
Improving disease prediction
Previous disease risk predictions indicated large regions, such as the whole of southern Europe, had a similar risk of local dengue cases over long timeframes, leaving local authorities uncertain whether their area was likely to be affected and action to prevent outbreaks was necessary.
But the UKCEH-led advanced high-resolution modelling divides Europe as well as eastern Asia and North America into 10km squares, providing daily risk assessments for each area. The new research involves a more detailed study of the entire life cycle of a mosquito, examining the impact of local climate and competition for food on species traits such as how long it lives and the number of eggs it lays.
This provides more accurate assessments of where, when and for how long there are likely to be local dengue cases.
The new modelling has already correctly predicted the locations of several towns that have gone on to have their first dengue outbreaks this year including La Colle-sur-Loup, Baho and Montpellier-Pérols in southern France, and Vila-seca, north-east Spain.
We predict that the parts of Europe most affected by dengue fever will continue to be southern France and northern Italy, due to a favourable climate, a stable mosquito population and the high number of travellers returning from tropical countries where the disease is prevalent. However, areas of risk are expanding northwards. Our ongoing research is modelling of the likely extent of future outbreaks in Europe under future climate change.
Dr Dominic Brass, lead author
UK Centre for Ecology & Hydrology, Wallingford, Oxfordshire, UK.
Guiding action
The study team, from UKCEH, University of Glasgow, University of Reading, and Biomathematics and Statistics Scotland, hopes its more accurate predictions will guide preventative measures in areas that are at risk of dengue outbreaks. Possible action includes checking areas where mosquitoes typically lay their eggs such as standing water, as happened at the Paris Olympics, as well as advising the public to protect themselves against bites.**
Our novel modelling technique shows remarkable accuracy for identifying the seasonal patterns and mechanisms of the risk of dengue fever outbreaks, providing a valuable tool for authorities drawing up action plans to prevent and control the spread of the disease.
Dr Steven White, senior author
UK Centre for Ecology & Hydrology, Wallingford, Oxfordshire, UK.
Rapid spread
In regions where Asian tiger mosquitoes are already established – including 13 countries in Europe – it is invaluable to know the times of year when there is greatest risk of a dengue fever outbreak, and how severe the risk is.
For other areas, it is useful to know what the disease risk would be if the species did arrive. This change can happen fairly quickly; Paris, for example, went from the first detection of the tiger mosquito in 2015 to having a dengue outbreak last year.
So far, the French capital is the most northerly place in Europe where there has been a local outbreak of dengue fever, and it also had a locally acquired (autochthonous) case of chikungunya disease in July after someone was bitten by an infected Asian tiger mosquito.
The UKCEH researchers say the modelling principles used in the dengue fever study can also be applied to other diseases transmitted by the tiger mosquito, such as chikungunya or Zika. They are currently drawing up risk maps for these diseases.
Asian tiger mosquito eggs have been found in south-east England, but the species has not yet become established in the UK. However, the research team warns this is likely to change in the future as the UK feels the effects of climate change.
Paper informationBrass et al. 2024. Phenotypic plasticity in vector traits drives trends in global disease incidence: the spread of dengue virus by Aedes albopictus. Nature Communications. DOI: 10.1038/s41467-024-52144-5. (Open access).
Notes:*About dengue fever
Dengue is a viral infection for which there is no specific treatment, though symptoms can usually be treated by general medicines. Most people are either asymptomatic or have flu-like conditions but in some people get severe muscle and joint pain, and in some cases, it causes internal bleeding and can be fatal.
Since the beginning of 2024, over 12 million dengue cases and over 8,000 dengue-related deaths have been reported from 86 countries/territories, according to the ECDC. The total cases so far is already more than twice the number of cases reported throughout 2023
Globally, the disease is mainly spread by Yellow fever mosquito (Aedes aegypti), which transmits several viruses but is now largely absent from Europe.
Why are Asian tiger mosquitoes spreading?
Tiger mosquitoes are native to the tropical forests of south-east Asia but in the past 50 years have expanded their range to the Americas, Africa, the Middle East and Europe due the global movement of people and trade. Their eggs can become concealed within used tyres imported from Asia, and adult mosquitoes can be moved from one area to another by human transport.
A warmer climate in northern hemisphere countries means the mosquitoes’ eggs are more likely to survive, thereby increasing populations, and hotter temperatures cause viruses to replicate faster.
What is being done to tackle the species and dengue fever?
Effective early diagnosis of conditions provides a warning about a local outbreak, so authorities can communicate the risks to the public.
**Personal measures to reduce the risk of bites include wearing clothes that cover most of the body as well as the use of mosquito repellent and screens over windows and doors. People in at-risk areas are advised be aware of potential mosquito breeding grounds, particularly standing water, including ensuring air conditioner drains are free of water, keeping bins covered to prevent them gathering rain and regularly changing water in birdbaths
Measures by local authorities to tackle dengue fever outbreaks when they occur include fumigation of potential mosquito breeding sites, open spaces and infected people’s homes.
In Northern Europe, the focus is on preventing further expansion of Asian tiger mosquitoes through surveillance, particularly around container ports and other major goods corridors.
AbstractAlthough the scientists attribute the increasing range of this mosquito vector for dengue fever and other infections such as zika, to 'phenotypic 'plasticity' in response to environmental change, in other words to evolution under environmental selection pressure, creationists must reject this by dogma as an article of cult belief. Nor can they blame 'genetic entropy' and the idiotic nonsense of 'devolution' because the changes are manifestly benefitting the mosquitos, and the viruses are riding on the back of that success.
The incidence of vector-borne disease is on the rise globally, with burdens increasing in endemic countries and outbreaks occurring in new locations. Effective mitigation and intervention strategies require models that accurately predict both spatial and temporal changes in disease dynamics, but this remains challenging due to the complex and interactive relationships between environmental variation and the vector traits that govern the transmission of vector-borne diseases. Predictions of disease risk in the literature typically assume that vector traits vary instantaneously and independently of population density, and therefore do not capture the delayed response of these same traits to past biotic and abiotic environments. We argue here that to produce accurate predictions of disease risk it is necessary to account for environmentally driven and delayed instances of phenotypic plasticity. To show this, we develop a stage and phenotypically structured model for the invasive mosquito vector, Aedes albopictus, and dengue, the second most prevalent human vector-borne disease worldwide. We find that environmental variation drives a dynamic phenotypic structure in the mosquito population, which accurately predicts global patterns of mosquito trait-abundance dynamics. In turn, this interacts with disease transmission to capture historic dengue outbreaks. By comparing the model to a suite of simpler models, we reveal that it is the delayed phenotypic structure that is critical for accurate prediction. Consequently, the incorporation of vector trait relationships into transmission models is critical to improvement of early warning systems that inform mitigation and control strategies.
Introduction
Vector-borne diseases (VBDs) are primarily vectored by ectothermic arthropods, whose life history is sensitive to environmental variation1. Our ability to predict if vector populations can sustain pathogen transmission across the species range requires an understanding of how environmental variation and vector life-history interact2,3. There is now a rich literature exploring the mechanisms through which environmental variation alters vector trait expression, but explicit and delayed mechanisms of individual variation are generally omitted, even in extensively studied systems4. For example, adult mosquitoes experiencing hot, dry summer conditions have a shorter lifespan than adults that are subject to more favourable temperatures5. Short-lived mosquitoes are less likely to survive to complete the extrinsic incubation period and take a subsequent blood meal to transmit the pathogen. Therefore, there is environmentally driven inter-annual and inter-regional variation in the ability of mosquitoes to vector disease6.
Predictions of the relative risk of VBDs, via metrics such as the basic reproduction number, are highly sensitive to traits such as adult longevity. Seasonal and regional variation in this trait is often accounted for by parameterising functions that directly relate the current temperature to the longevity of adult mosquitoes7,8. However, adult longevity has also been shown to vary in response to an individual’s experience of competition and temperature during development. Therefore, the traits expressed by individuals in the population result from complex interactions between past environmental conditions and population states9. By ignoring the role that a vector’s historic environmental experiences have on biological traits critical to disease transmission we implicitly make the mean-field assumption, that all individuals are equally competent vectors regardless of their past experiences of the biotic and abiotic environment10. This mean-field assumption compromises our ability to assess the risk of vector-borne disease in variable environments, where seasonal patterns in environmental variables induce seasonal variation in vector population dynamics. Similarly, mean-field assumptions limit our ability to assess the relative risk of VBDs between climatic zones, such as between tropical and temperate environments, where different patterns of environmental variation occur.
The delayed effect of developmental experience on adult traits is an example of phenotypic plasticity, the ability of organisms expressing the same genotype to express different phenotypes according to the environmental conditions they are subject to11. To demonstrate how mechanisms of delayed phenotypic plasticity can be incorporated into predictions of VBDs we consider the ability of the invasive mosquito species Aedes albopictus to vector dengue virus. We use a recently derived phenotypically explicit mathematical modelling framework to represent how historical biotic and abiotic environmental conditions determine the trait dynamics of vector populations12. Ae. albopictus is a competent vector of dengue virus that is now widely distributed in temperate zones into which dengue is regularly imported13. Dengue is a viral VBD that has seen a recent dramatic increase in cases: in 2000 there were about half a million cases, which has risen to over 4.2 million in 2022, caused by a combination of climate and anthropogenic change14.
Despite predictions of broad suitability for the transmission of dengue virus by Ae. albopictus in temperate climates such as Europe by both statistical and mechanistic modelling approaches, outbreaks in these regions have so far been limited in size and duration15,16. This mismatch between observed and predicted disease incidence motivates the development of modelling approaches that are better able to reflect the currently observed global incidence of dengue. Due to the species' global range (currently between 0 and 52.5 degrees latitude13), Ae. albopictus populations are subject to a diverse range of environmental conditions. Environmental variation has been shown to induce variable trait dynamics in field populations and this may alter the ability of populations in different environments to vector disease17,18. It is our hypothesis that, by accounting for the effect of biotic and abiotic environmental variation on the phenotypic trait structure and population dynamics of Ae. albopictus, both the population dynamics of the species across its range and the current patterns of disease incidence around the globe can be better understood. Further, we propose that omitting mechanisms of phenotypic plasticity limits the ability of models to produce accurate and generalisable predictions of disease incidence.Fig. 4: Geographic variation in mosquito density and suitability for disease transmission. A, C, E The average adult density during the active season of Ae. albopictus in North America, Europe, and Asia respectively between the years 2010−2019. B, D, F The average number of months the reproduction number was greater than one (Rt > 1) in North America, Europe, and Asia respectively between the years 2010−2019. In D the yellow circles are centred on regions within which autochthonous dengue transmission was detected between the years 2010 − 2019 and the red circles indicate the locations of outbreaks after this period. Source data are provided as a Source Data file.
A, C, E The average adult density during the active season of Ae. albopictus in North America, Europe, and Asia respectively between the years 2010−2019. B, D, F The average number of months the reproduction number was greater than one (Rt > 1) in North America, Europe, and Asia respectively between the years 2010−2019. In D the yellow circles are centred on regions within which autochthonous dengue transmission was detected between the years 2010 − 2019 and the red circles indicate the locations of outbreaks after this period. Source data are provided as a Source Data file.
Brass, D.P., Cobbold, C.A., Purse, B.V. et al. Role of vector phenotypic plasticity in disease transmission as illustrated by the spread of dengue virus by Aedes albopictus. Nat Commun 15, 7823 (2024). https://doi.org/10.1038/s41467-024-52144-5
Copyright: © 2024 The authors.
Published by Springer Nature Ltd. Open access.
Reprinted under a Creative Commons Attribution 4.0 International license (CC BY 4.0)
So, creationist dogma means they must attribute these changes to the intentional design of their putative intelligent [sic] designer, the only conclusion being that their putative designer is a pestilential malevolence, forever trying to find new ways to increase the suffering in the world and make life unpleasant for its creation.
Or they could turn their back on cult dogma and attribute the whole thing to evolution, in accordance with the Theory of Evolution by Natural Selection.
The Malevolent Designer: Why Nature's God is Not Good
Illustrated by Catherine Webber-Hounslow.
The Unintelligent Designer: Refuting The Intelligent Design Hoax




No comments:
Post a Comment
Obscene, threatening or obnoxious messages, preaching, abuse and spam will be removed, as will anything by known Internet trolls and stalkers, by known sock-puppet accounts and anything not connected with the post,
A claim made without evidence can be dismissed without evidence. Remember: your opinion is not an established fact unless corroborated.