Nematodes May Cause Disease Indirectly, via the Viruses They Carry
Not content with a whole range of parasitic worms to cause suffering, creationism's divine malevolence has also designed them to carry a payload of viruses to make their victims suffer even more.
This is the inescapable conclusion an honest creationist (if there is such a thing) would need to reach if he/she made an honest assessment of the facts just published by a team of researchers from the Centre for Neglected Tropical Diseases, Liverpool School of Tropical Medicine (LSTM), Liverpool, UK and the Global Health Institute, University of Antwerp, Antwerp, Belgium, in the journal Nature Microbiology.
This research team have identified a wide variety of viruses infecting nematodes and, most worryingly, the parasitic nematodes responsible for diseases in humans, so it is entirely possible that the diseases associated with infection by these nematodes could actually be caused by the viruses they carry. Related nematodes also show signs of virus-nematode co-evolution over millions of years in the form of a global distribution of nematode-virus associations.
Their research is explained in a press release from LSTM:
Tell me all about parasitic nematodes and the diseases they cause in humans, please. Parasitic nematodes (roundworms) are a diverse group of organisms that infect humans, animals, and plants. They can be found in various environments, from soil to water, and have adapted to parasitize a wide range of hosts. These nematodes can cause various diseases in humans, ranging from mild discomfort to severe and life-threatening conditions.
Overview of Parasitic Nematodes
Parasitic nematodes are classified under the phylum Nematoda. They are elongated, cylindrical, and non-segmented worms. Some of the most common parasitic nematodes that infect humans include:
- Ascaris lumbricoides (giant roundworm)
- Trichuris trichiura (whipworm)
- Ancylostoma duodenale and Necator americanus (hookworms)
- Strongyloides stercoralis (threadworm)
- Enterobius vermicularis (pinworm)
- Wuchereria bancrofti and Brugia malayi (filarial worms)
- Onchocerca volvulus (cause of river blindness)
- Dracunculus medinensis (Guinea worm)
- Toxocara spp (Toxocariasis)
Common Diseases Caused by Parasitic Nematodes in Humans
- Ascariasis
- Causative agent: Ascaris lumbricoides
- Symptoms: Often asymptomatic, but heavy infections can cause abdominal pain, malnutrition, growth retardation in children, intestinal obstruction, and respiratory issues (due to larval migration through the lungs).
- Transmission: Ingestion of eggs from contaminated soil, water, or food.
- Geographical distribution: Common in tropical and subtropical areas with poor sanitation.
- Trichuriasis (Whipworm Infection)
- Causative agent: Trichuris trichiura
- Symptoms: Asymptomatic in mild cases, but heavy infections can cause diarrhea, rectal prolapse, anemia, growth retardation in children, and malnutrition.
- Transmission: Ingestion of eggs from contaminated soil, food, or water.
- Geographical distribution: Widespread in tropical regions, especially in areas with poor sanitation.
- Hookworm Infections
- Causative agents: Ancylostoma duodenale and Necator americanus
- Symptoms: Anemia (due to blood loss in the intestines), abdominal pain, diarrhea, weight loss, and fatigue. Skin itching and rash can occur where larvae penetrate the skin.
- Transmission: Larvae penetrate the skin, usually through bare feet when walking on contaminated soil.
- Geographical distribution: Common in warm, moist climates, particularly in sub-Saharan Africa, Southeast Asia, and South America.
- Strongyloidiasis
- Causative agent: Strongyloides stercoralis
- Symptoms: Often asymptomatic, but can cause abdominal pain, diarrhea, skin rash, and severe life-threatening hyperinfection syndrome, especially in immunocompromised individuals.
- Transmission: Larvae penetrate the skin from contact with contaminated soil.
- Geographical distribution: Tropical and subtropical regions, but also occurs in temperate areas.
- Enterobiasis (Pinworm Infection)
- Causative agent: Enterobius vermicularis
- Symptoms: Perianal itching, irritability, sleep disturbances, and sometimes abdominal pain.
- Transmission: Ingestion of eggs from contaminated hands, surfaces, or food.
- Geographical distribution: Worldwide, particularly in temperate regions; commonly affects children.
- Lymphatic Filariasis (Elephantiasis)
- Causative agents: Wuchereria bancrofti, Brugia malayi, Brugia timori
- Symptoms: Lymphedema (swelling of limbs), elephantiasis (thickening of the skin and underlying tissues), and hydrocele (swelling of the scrotum).
- Transmission: Mosquito bites (primarily Culex, Anopheles, and Aedes species).
- Geographical distribution: Sub-Saharan Africa, Southeast Asia, the Pacific, and parts of the Americas.
- Onchocerciasis (River Blindness)
- Causative agent: Onchocerca volvulus
- Symptoms: Severe itching, skin rash, depigmentation, and nodules under the skin. Can lead to visual impairment or blindness if the eyes are affected.
- Transmission: Blackfly bites (genus Simulium).
- Geographical distribution: Sub-Saharan Africa, with some cases in Latin America and Yemen.
- Dracunculiasis (Guinea Worm Disease)
- Causative agent: Dracunculus medinensis
- Symptoms: Painful blister and ulcer, often on the lower limbs. The emerging worm can cause secondary infections.
- Transmission: Ingestion of water contaminated with infected copepods (small crustaceans) that harbor larvae.
- Geographical distribution: Historically widespread in Africa and Asia, now largely confined to a few countries due to eradication efforts.
- Toxocariasis
- Causative agents: Toxocara canis and Toxocara cati
- Symptoms: In visceral larva migrans (VLM), symptoms may include fever, cough, abdominal pain, and eosinophilia; in ocular larva migrans (OLM), it may cause visual impairment or blindness.
- Transmission: Ingestion of eggs from contaminated soil or food, or from contact with infected animal feces.
- Geographical distribution: Worldwide, especially in areas where pets are not regularly dewormed.
Diagnosis and Treatment
Diagnosis of parasitic nematode infections typically involves stool examinations for eggs or larvae, blood tests for antibodies or microfilariae, or skin snips in the case of onchocerciasis. Advanced imaging or biopsies may be required for some infections.
Treatment often involves anti-parasitic medications such as albendazole, mebendazole, ivermectin, diethylcarbamazine, and praziquantel, depending on the type of nematode and the severity of the infection.
Prevention and Control
- Improving sanitation: Proper disposal of human feces and access to clean water.
- Personal hygiene: Handwashing, wearing shoes, and using protective clothing.
- Mass drug administration (MDA): In endemic areas to reduce transmission, especially for lymphatic filariasis and onchocerciasis.
- Vector control: Reducing the population of mosquitoes and blackflies.
- Public health education: Raising awareness about transmission and prevention methods.
Would you like more information on any specific nematode or disease?
Newly discovered viruses in parasitic nematodes could change our understanding of how they cause disease
New research shows that parasitic nematodes, responsible for infecting more than a billion people globally, carry viruses that may solve the puzzle of why some cause serious diseases.
A study led by Liverpool School of Tropical Medicine (LSTM) used cutting-edge bioinformatic data mining techniques to identify 91 RNA viruses in 28 species of parasitic nematodes, representing 70% of those that infect people and animals. Often these are symptomless or not serious, but some can lead to severe, life-changing disease.
Nematode worms are the most abundant animals on the planet, prevalent in all continents worldwide, with several species infecting humans as well as agriculturally and economically important animals and crops. And yet in several cases, scientists do not know how some nematodes cause certain diseases.
The new research, published in Nature Microbiology(link is external)(opens in a new tab), opens the door to further study of whether these newly discovered viruses – only five of which were previously known to science – could contribute to many chronic, debilitating conditions. If a connection can be proven, it could pave the way for more effective treatments in the future.
This is a truly exciting discovery and could change our understanding of the millions of infections caused by parasitic nematodes. Finding an RNA virus in any organism is significant, because these types of viruses are well-known agents of disease. When these worms that live inside of us release these viruses, they spread throughout the blood and tissues and provoke an immune response. This raises the question of whether any of the diseases that these parasites are responsible for could be driven by the virus rather than directly by the parasitic nematode.
Professor Mark J. Taylor, co-corresponding author
Professor of Parasitology
Centre for Neglected Tropical Diseases
Department of Tropical Disease Biology
Liverpool School of Tropical Medicine, Liverpool, UK.
Parasitic nematodes including hookworms and whipworms can cause severe abdominal problems and bloody diarrhoea, stunted development and anaemia. Infection with filarial worms can lead to disfiguring conditions such as lymphoedema or ‘elephantiasis’, and onchocerciasis, or ‘river blindness’, that leads to blindness and skin disease.
This is a truly exciting discovery and could change our understanding of the millions of infections caused by parasitic nematodes.
The study authors propose that these newly identified viruses may play a role in some of these conditions. For example, Onchocerciasis-Associated Epilepsy (OAE) that occurs in children and adolescents in Sub-Saharan Africa has recently been associated with onchocerciasis, but it is not known why this causes neurological symptoms such as uncontrollable repeated head nodding, as well as severe stunting, delayed puberty and impaired mental health.
One of the viruses in the parasites that cause onchocerciasis identified in the new study is a rhabdovirus – the type that causes rabies. The authors of the study suggest that if this virus is infecting or damaging human nerve or brain tissue, that could explain the symptoms of OAE.
The full extent and diversity of the viruses living in parasitic nematodes, how they impact nematode biology and whether they act as drivers of disease in people and animals now requires further study.
The illuminating discovery of these widespread yet previously hidden viruses was first made by Dr Shannon Quek, a Postdoctoral Research Associate at LSTM and lead author of the new study, who had initially been using the same data mining method to screen for viruses within mosquitoes that spread disease, before deciding to investigate nematodes.
As a child [in Indonesian], I saw a lot of people infected with these diseases and I suffered from the dengue virus on three occasions. That got me interested in tropical diseases. Diseases caused by parasitic nematodes are very long-term, life-long illnesses that persistently affect people. It has a significant impact on people's quality of life, their economic outputs and mental health.
There are a lot of studies about the microbiomes of mosquitoes, and how the bacteria that lives inside can block the spread of viruses, which might stop vector-borne diseases like dengue. This interplay between organisms in the same host led me to think - what else might be inside parasitic nematodes as well? Which after my discovery will now be the focus of our research.
Dr Shannon Quek, lead author
Centre for Neglected Tropical Diseases
Department of Tropical Disease Biology
Liverpool School of Tropical Medicine, Liverpool, UK.
The study also involved researchers from University of Antwerp and KU Leuven, Belgium, Brock University, Canada, University of Queensland, Australia, University of Buea, Cameroon and the University of Energy and Natural Resources, Ghana.
Abstract
Parasitic nematodes have an intimate, chronic and lifelong exposure to vertebrate tissues. Here we mined 41 published parasitic nematode transcriptomes from vertebrate hosts and identified 91 RNA viruses across 13 virus orders from 24 families in ~70% (28 out of 41) of parasitic nematode species, which include only 5 previously reported viruses. We observe widespread distribution of virus–nematode associations across multiple continents, suggesting an ancestral acquisition event and host–virus co-evolution. Characterization of viruses of Brugia malayi (BMRV1) and Onchocerca volvulus (OVRV1) shows that these viruses are abundant in reproductive tissues of adult parasites. Importantly, the presence of BMRV1 RNA in B. malayi parasites mounts an RNA interference response against BMRV1 suggesting active viral replication. Finally, BMRV1 and OVRV1 were found to elicit antibody responses in serum samples from infected jirds and infected or exposed humans, indicating direct exposure to the immune system.
Main
Humans and animals are frequently infected with multiple species of parasitic nematodes1,2,3 and suffer from chronic, lifelong infections and exposure to continuous reinfection4. Such infections impose a substantial health burden on billions of people, impacting their health, quality of life and economic productivity. Medically important parasitic nematodes infect over one billion people, resulting in up to 7.53 million disability-adjusted life years globally5. Prominent examples include intestinal species such as Ascaris lumbricoides and Trichuris trichiura4, which infect an estimated 511 and 412 million people, respectively5, as well as the hookworms Necator americanus, Ancylostoma duodenale and Ancylostoma ceylanicum, which collectively infect up to 186 million people globally5. Infected individuals can suffer from severe abdominal discomfort, bloody diarrhoea, stunted development and anaemia. Other examples include the filarial nematodes Wuchereria bancrofti and Brugia malayi, the causative agents of lymphatic filariasis that infect up to 96 million people globally5,6, and Onchocerca volvulus, which infects up to 21 million people5. In the case of O. volvulus, recent estimates indicate that 14.6 million are afflicted with skin disease and 1.15 million with blindness7. Furthermore, there has been increasing recognition of a disease known as onchocerciasis-associated epilepsy (OAE), occurring in children and adolescents in onchocerciasis meso- and hyperendemic foci across sub-Saharan Africa8. This condition manifests as a variety of epileptic seizures, including uncontrollable repeated head nodding (‘nodding syndrome’), as well as severe stunting, delayed puberty and impaired mental health (Nakalanga syndrome)9. OAE has been epidemiologically linked to infection with O. volvulus10, but the pathogenesis has yet to be identified8.
A variety of viruses can be found infecting several human parasitic protozoa, including Plasmodium vivax, Trichomonas vaginalis and Cryptosporidium parvum11,12. Viruses infecting Leishmania sp. have been studied in great detail13 and can increase disease severity, parasite prevalence and potentially the incidence rates of both drug resistance and mucocutaneous leishmaniasis14,15. RNA virus infections have been identified in plant-parasitic nematodes16, parasitic flatworms17,18 and free-living nematodes17,19,20, although the impact of viral infections on the biology of the worms is largely unknown.
Here we analysed the transcriptomes of 41 parasitic nematode species infecting humans and animals and discovered 91 virus or virus-like genomic sequences across 28 species. We further characterize the viruses infecting B. malayi and O. volvulus, describing their genomic diversity, geographic spread, phylogeny, abundance throughout different developmental stages, tissue tropism, localization and vertebrate host serology. Finally, we show that an RNA interference (RNAi) response is induced in B. malayi against BMRV1, providing evidence for active viral replication.
[…]
Discussion
We reveal an abundant and diverse RNA virome spanning 14 different viral orders and 24 families within parasitic nematodes. Of the 91 viruses discovered, only 5 have been previously reported, including 3 from A. suum and A. lumbricoides23,25. Our survey is probably an under-representation of the true extent and diversity of the parasitic nematode RNA virome owing to a variety of factors including variations in sample preparation resulting in discarded viral reads and the restricted number, or lack, of transcriptomes for several important parasites. Nevertheless, our analysis supports a conserved global spread of virus–nematode associations across multiple continents in the case of the viruses of A. suum and A. lumbricoides, and O. volvulus, suggesting an ancient and stable co-evolution. This is perhaps best exemplified by members of the Trichinellidae (Supplementary Fig. 1), which show a close evolutionary relationship, as well as phylogenetic clustering of diverse virus sequences from different species and orders of parasitic nematodes.
The parasitic nematodes identified with viruses include several important human parasitic nematodes, A. lumbricoides, T. trichiura, O. volvulus, B. malayi, A. ceylanicum and Trichinella spiralis, which cause substantial public health issues, with over 1.5 billion people infected with one or more such parasites4,5,6,44,45. Several other species cause an even greater global burden in the livestock industry46, with 15 economically important parasites (A. suum, Dictyocaulus viviparous, Haemonchus contortus, Ostertagia ostertagi, Oesophagostomum dentatum, Teladorsagia circumcinta, Trichuris suis plus 8 Trichinella spp.) of cattle, sheep and pigs, harbouring 37 previously unreported viruses.
The full extent and diversity of the parasitic nematode RNA virome, how it impacts nematode biology and whether they act as drivers or modulators of disease pathogenesis remain critical knowledge gaps. Indeed, in the parasite Toxocara canis, which causes neurotoxocariasis, components of the TCLA virus have been reported to be highly expressed in infective larvae (18% of expressed sequence tags) before entry into a vertebrate host (for example, humans and dogs)29, with human infections eliciting antibody responses against several TCLA virus proteins29, indicating potential roles in transmission and infectivity. Alternatively, extrapolation from the most well-characterized RNA viruses of Leishmania sp. protozoan parasites suggests potential roles of nematode viruses in disease pathology and progression. Both Leishmania virus 1 (LRV1) and T. vaginalis virus induce hyperinflammatory immunity, which drives disease pathogenesis and subverts host immunity to the parasites’ advantage14,15,47. We show that BMRV1 and OVRV1 elicit antibody responses from the host showing direct exposure to the immune system, and we speculate that this suggests the potential to modulate host immunity to the parasite and cross-reactive immunity to other RNA viruses.
[…]
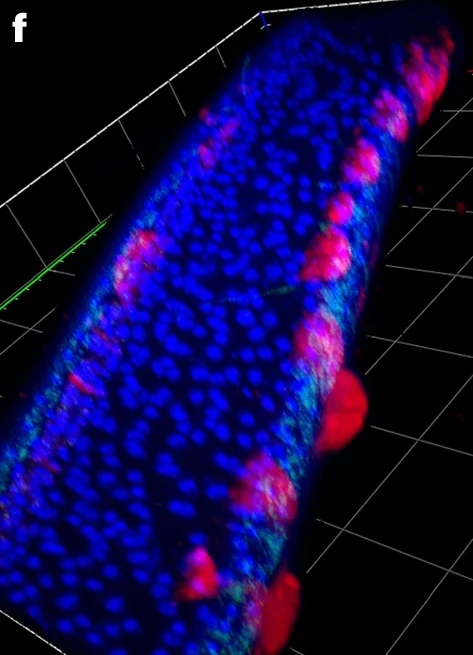
Fig. 4: Representative FISH microscopy images of B. malayi showing localization of virus RNA within nematode tissues, alongside the Wolbachia endosymbiont as a technical control.Virus RNA stained red; Wolbachia stained green; DAPI nuclear stain blue. a–e, Note the different levels of viral infection in microfilariae (a), localization of the viral stain in male testes (b) and the hypodermal cells near the male spicule (c). Virus signal within adult female reproductive tracts appears between developing eggs within the paired uteri of adult females, with early embrys in the left uteri and ‘pretzel-stage’ microfilariae in the right (d), with the developing eggs casting a ‘shadow’ in between virus staining, visible in 3D images of female uteri (e). f–j, In older adults (>12 months), we observed ‘epicuticular inflations’ often with an intense viral signal (f), typically occurring near the head (g) or tail regions of the nematodes. They can appear as single separate inflations at different nematode orientations, either next to internal organs (h) or the hypodermal chords (i), or as a continuous inflation along the nematode flank (j). Scale bars measure 20 µm (a,b,h,i) or 50 µm (d,g). Gridlines in three-dimensional 𝓏-stack figures (c,e,f,j) measure 40 µm by 40 µm. A total of 15 adult male and female parasites were processed in separate experiments. Parasites with epicuticular inflations were typically between 12 and 19 months at the time of sampling, with jird animal hosts being 15–22 months of age, respectively. Parasites without were typically 3–6 months of age, with the jird animal hosts being 6–9 months of age.
Fig. 5: Validation of OVRV1 using RT-PCR, western blot and representative IFA staining of O. volvulus nodules with anti-OVRV1 glycoprotein antibodies. Anti-OVRV1 glycoprotein antibodies stained green; DAPI nuclear stain blue. a, RT-PCR experiments show that OVRV1 can be amplified only from reverse-transcribed RNA, from both O. volvulus (lane 1, n = 1) and O. ochengi (lanes 2–4, n = 3). b, Western blots against the OVRV1 glycoprotein show different molecular weight bands occurring depending on the life cycle stage of O. volvulus (n = 3). All IFA images include the DAPI nuclear stain (blue). c,d, Images of the paired uteri from adult O. volvulus females show virus stains surrounding and entering developing embryos within the uteri (solid arrow), while surrounding but not within the early embryos (hollow arrow). Developing embryos can show either complete infection rates (c) or a much smaller proportion (d). e, Mature microfilariae released from the female, located within surrounding nodule tissues, stain heavily for OVRV1 glycoprotein. f,g, Intense antibody staining is observed surrounding the nematode rachis, where eggs are first formed (solid arrows). The heavily stained rachis is either surrounded by early-stage eggs with green staining surrounding them (f) or without surrounding eggs (g). h,i, Cellular inflations containing intense antibody staining are observed on the external face of the adult female uterine walls (solid arrows). j,k, Male O. volvulus are frequently observed to be infected, with viral stains occurring in different tissues (j), as well as surrounding and entering the male testes (k). Parasites were obtained from sections of fixed O. volvulus nodules from human patients (n = 8 nodules).
Anti-OVRV1 glycoprotein antibodies stained green; DAPI nuclear stain blue. a, RT-PCR experiments show that OVRV1 can be amplified only from reverse-transcribed RNA, from both O. volvulus (lane 1, n = 1) and O. ochengi (lanes 2–4, n = 3). b, Western blots against the OVRV1 glycoprotein show different molecular weight bands occurring depending on the life cycle stage of O. volvulus (n = 3). All IFA images include the DAPI nuclear stain (blue). c,d, Images of the paired uteri from adult O. volvulus females show virus stains surrounding and entering developing embryos within the uteri (solid arrow), while surrounding but not within the early embryos (hollow arrow). Developing embryos can show either complete infection rates (c) or a much smaller proportion (d). e, Mature microfilariae released from the female, located within surrounding nodule tissues, stain heavily for OVRV1 glycoprotein. f,g, Intense antibody staining is observed surrounding the nematode rachis, where eggs are first formed (solid arrows). The heavily stained rachis is either surrounded by early-stage eggs with green staining surrounding them (f) or without surrounding eggs (g). h,i, Cellular inflations containing intense antibody staining are observed on the external face of the adult female uterine walls (solid arrows). j,k, Male O. volvulus are frequently observed to be infected, with viral stains occurring in different tissues (j), as well as surrounding and entering the male testes (k). Parasites were obtained from sections of fixed O. volvulus nodules from human patients (n = 8 nodules).
Quek, S., Hadermann, A., Wu, Y. et al.
Diverse RNA viruses of parasitic nematodes can elicit antibody responses in vertebrate hosts. Nat Microbiol (2024). https://doi.org/10.1038/s41564-024-01796-6
Copyright: © 2024 The authors.
Published by Springer Nature. Open access.
Reprinted under a Creative Commons Attribution 4.0 International license (CC BY 4.0)
So, if you reject the evolutionary explanation of these viruses-nematode associations in favour of a creationist 'intelligent [sic] design' explanation you must assume the designer intended the consequences of its design since it is axiomatic of the creationist cult that the designer is a perfect, omniscient god for whom the consequences of its design must have been known in advance and so were designed with that function in mind.
And of course we can dismiss the childish nonsense about 'genetic entropy; causing 'devolution' [sic] because these viruses are clearly gaining an advantage in infecting the nematodes because that gives them easy access to their vertebrate hosts, and anything which conveys an advantage is evolution, not 'devolution'. Only someone ignorant of evolution would fall for such biologically nonsensical excuse for parasites, as any biologist worthy of the term would have known before he came up with it.
So, the question remains unanswered by creationists - is this an example of malevolent design, or of evolution?
The Malevolent Designer: Why Nature's God is Not Good
Illustrated by Catherine Webber-Hounslow.
The Unintelligent Designer: Refuting The Intelligent Design Hoax




No comments:
Post a Comment
Obscene, threatening or obnoxious messages, preaching, abuse and spam will be removed, as will anything by known Internet trolls and stalkers, by known sock-puppet accounts and anything not connected with the post,
A claim made without evidence can be dismissed without evidence. Remember: your opinion is not an established fact unless corroborated.