This gene variant contributed to the dietary and physiological evolution of modern humans | ScienceDaily
One of the enduring claims in creationist circles is that mutations are invariably harmful—deleterious at best, fatal at worst—and thus incapable of driving evolutionary progress. This notion underpins Michael J. Behe's concept of "devolution," which posits that genetic changes represent a decline from an assumed state of original perfection. Yet, this perspective fails to account for how such "inferior" mutations could supplant their "perfect" predecessors in a population. Moreover, if a genome were truly perfect, it would replicate without error, precluding any mutations—and, by extension, any evolutionary change.
A recent study published in Cell Genomics challenges this dogma by identifying a regulatory variant of the ACSF3 gene that appears to have played a significant role in human evolution . This variant, known as rs34590044-A, enhances the expression of ACSF3 in the liver, influencing both stature and basal metabolic rate (BMR). Notably, the effects of this mutation are amplified in individuals consuming meat-rich diets, suggesting a link between genetic adaptation and dietary shifts in human history.[1.1,2.1]
The ACSF3 gene encodes an enzyme involved in mitochondrial fatty acid synthesis, a critical process for energy metabolism. Increased expression of ACSF3 has been associated with improved mitochondrial function and bone formation, potentially contributing to greater height and higher BMR in modern humans. These findings underscore the role of beneficial mutations in human adaptation and evolution, directly contradicting the creationist assertion that mutations cannot produce advantageous traits.[3.1,4.1]
This discovery not only provides insight into the genetic factors influencing human physiology but also exemplifies how mutations can facilitate evolutionary advancements, thereby challenging the notion that all genetic changes are inherently detrimental.
The team from Human Phenome Institute, Zhongshan Hospital and School of Life Sciences, Fudan University, Shanghai, China with Mark Stoneking, Department of Evolutionary Genetics, Max Planck Institute for Evolutionary Anthropology, Leipzig, Germany, have recently published their findings in the open access Cell Press journal, Cell Genomics.
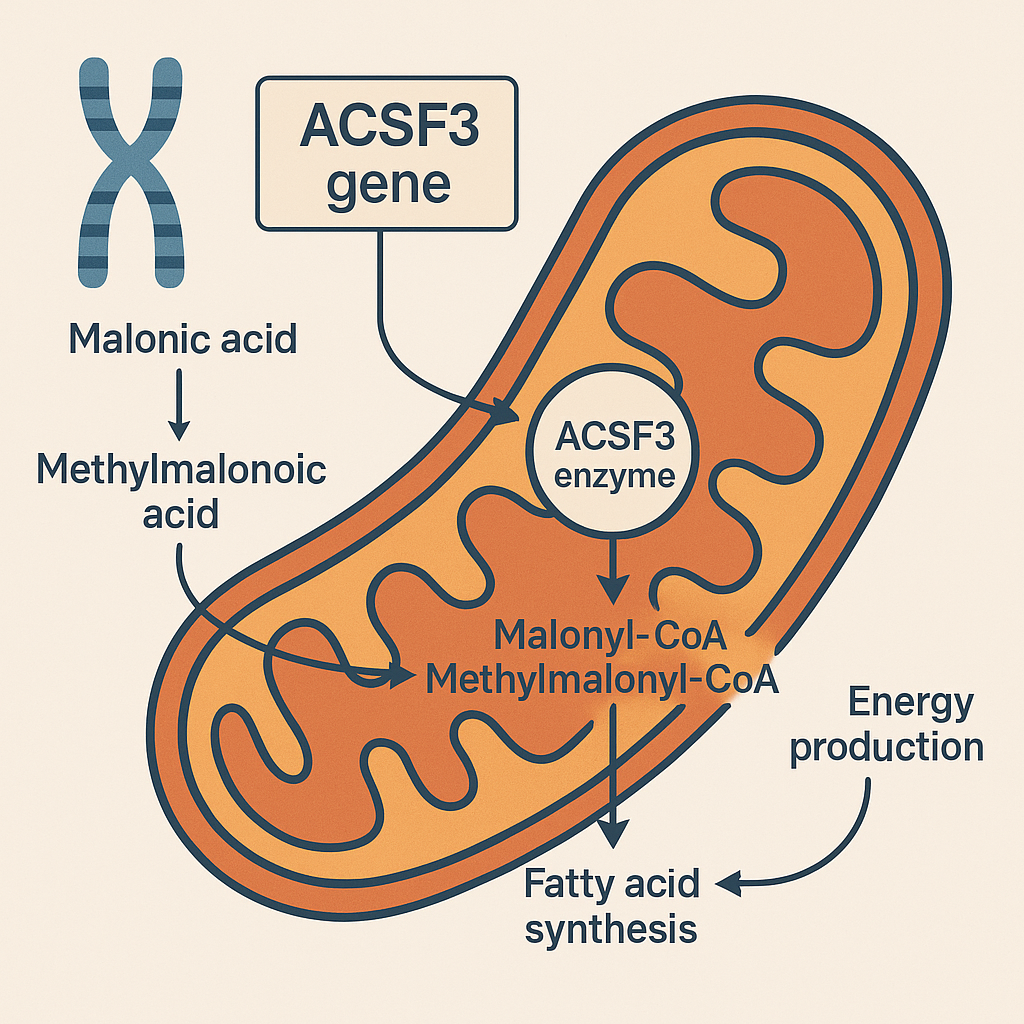
What information do you have on the gene ACSF3 ? The ACSF3 gene (Acyl-CoA Synthetase Family Member 3) encodes a mitochondrial enzyme pivotal in fatty acid metabolism and energy homeostasis. Located on chromosome 16q24.3, it comprises 17 exons and produces a 576-amino acid protein with a molecular weight of approximately 64.1 kDa.[1.2,2.2]It is also explained in information from Cell Press published in ScienceDaily:
Function in Mitochondrial Metabolism
ACSF3 functions as a malonyl-CoA synthetase, catalyzing the ATP-dependent conversion of malonic acid and methylmalonic acid into malonyl-CoA and methylmalonyl-CoA, respectively . These reactions are integral to the mitochondrial fatty acid synthesis (mtFAS) pathway, essential for producing lipoic acid—a cofactor necessary for several mitochondrial enzyme complexes involved in energy metabolism.[3.2,2.2]
Additionally, ACSF3-derived malonyl-CoA contributes to the post-translational modification of mitochondrial proteins through malonylation, influencing various metabolic processes.[4.2]
Clinical Significance
Mutations in ACSF3 are associated with **Combined Malonic and Methylmalonic Aciduria (CMAMMA)**, a rare metabolic disorder characterized by elevated levels of malonic and methylmalonic acids. Clinical manifestations range from developmental delays and hypotonia in children to neurological symptoms in adults.[3.2, 2.2]
Recent Research Insights
Emerging studies suggest that ACSF3 expression is influenced by feeding cycles, indicating a role in adapting mitochondrial metabolism to nutritional states . Furthermore, variations in ACSF3 expression have been linked to differences in lipid metabolism traits in livestock, highlighting its broader significance in energy regulation.[5.2]
This gene variant contributed to the dietary and physiological evolution of modern humans
Two of the traits that set modern humans apart from non-human primates are taller stature and a higher basal metabolic rate. Researchers have identified a genetic variant that contributed to the co-evolution of these traits. This mutation seems to help people grow taller -- especially when they consume a lot of meat.
The dietary shift from a primarily plant-based diet to increased meat consumption marks a major milestone in human evolution. Previous studies have suggested that this shift influenced many traits and phenotypes in anatomically modern humans. It is therefore not surprising that height may also have been affected.
Jin Li and He Huang, co-corresponding authors
Human Phenome Institute
Zhongshan Hospital and School of Life Sciences
Fudan University, Shanghai, China.
The researchers used the UK Biobank, a resource of biological samples and genomic data from 500,000 people, to identify genetic correlations between height and basal metabolic rate and found more than 6,000 potential causal variants. After narrowing down the likely functional impacts of these variants, including their effect on protein sequences and gene expression, a specific regulatory variant of ACSF3 emerged as particularly promising. Further experimentation revealed that the variant, called rs34590044-A, elevates ACSF3 expression in the liver of modern humans compared to other apes.
In anatomically modern humans, basal metabolic rate and stature exhibit notable evolutionary divergence compared to non-human apes. Although both traits, particularly height, have been extensively investigated, the evolutionary mechanisms driving these changes remain comparatively underexplored. That's why we decided to focus on these two traits together.
Shaohua Fan, co-corresponding author.
Human Phenome Institute
Zhongshan Hospital and School of Life Sciences
Fudan University, Shanghai, China.
To further validate these findings and determine how they relate to modern human traits, the team conducted detailed functional analyses of the variant and its effects on ACSF3 expression using both cellular and mouse models. Although the mechanism by which ACSF3 acts on the body is not fully understood, it appears to be localized to mitochondria, which the authors say explains its effects on metabolism. Increased expression of ACSF3 also appears to promote the formation of bone, which could contribute to increased height.
In a mouse model fed with the essential amino acids that are characteristic of meat-based diets, the researchers also noticed that when ACSF3 was overexpressed, the "meat" diet led to both increased body length and a higher basal metabolic rate.
The team notes that the ongoing study of global populations using a combination of approaches -- including multiomics, experimental technologies, computational algorithms, and diverse collections of ancient DNA -- is important for enhancing our understanding of complex evolutionary processes.
This research reveals the intricate interplay between the genetic, environmental, and demographic factors that have contributed to the emergence and evolution of anatomically modern humans. It also has important implications understanding susceptibility and resistance in contemporary metabolic disorders like type 2 diabetes, obesity, and metabolic syndrome.
Shaohua Fan.
The researchers expect that many more traits may have co-evolved through similar mechanisms. They plan to continue investigating the genetic basis of metabolic homeostasis in human evolution, with the aim of determining how human ancestors adapted to diverse diets throughout evolutionary history.
Publication:
HighlightsThis new finding places creationists in a particularly uncomfortable position. Their core argument has long been that mutations degrade genetic information and cannot result in new, functional adaptations. Yet the identification of a regulatory variant in the ACSF3 gene that appears to have been positively selected in human evolution directly contradicts this premise. Here we have a mutation that increases the expression of a gene involved in mitochondrial fatty acid metabolism—an enhancement that likely contributed to the greater height and higher basal metabolic rate observed in modern humans. This is not degradation; it is adaptation, and quite possibly an evolutionary advantage shaped by natural selection.
- A strong genetic correlation between height and basal metabolic rate in humans
- rs34590044-A is associated with increased height and basal metabolic rate
- rs34590044-A upregulates ACSF3 and controls amino acid metabolism
- rs34590044-A has been under positive selection in the last 20,000 years
Summary
Anatomically modern humans (AMHs) exhibit a significant increase in basal metabolic rate (BMR) and height compared to non-human apes. This study investigates the genetic basis underlying these traits. Our analyses reveal a strong genetic correlation between height and BMR. A regulatory mutation, rs34590044-A, was found to be associated with the increased height and BMR in AMHs. rs34590044-A upregulates the expression of ACSF3 by increasing its enhancer activity, leading to increased body length and BMR in mice fed essential amino acids which are characteristic of meat-based diets. In the British population, rs34590044-A has been under positive selection over the past 20,000 years, with a particularly strong signal in the last 5,000 years, as also evidenced by ancient DNA analysis. These results suggest that the emergence of rs34590044-A may have facilitated the adaptation to a meat-enriched diet in AMHs, with increased height and BMR as consequences of this dietary shift.
Introduction
The evolution of animals has been marked by profound morphological transformations that have enabled their adaptation to various environments. Among these adaptations, changes in body size and mass have been pivotal, shaping the evolutionary trajectories of numerous animal lineages and reflecting key innovations in mobility, thermoregulation, and resource acquisition.1 Studies have shown that changes in body mass and size are often accompanied by shifts in metabolic rate with basal metabolic rate (BMR) scaling as a power function of body mass.1,2,3,4 This allometric relationship has profound implications for understanding the energetic constraints and physiological limits that have shaped animal evolution.1,2,3,4
Over the past 6–15 million years, among many lineage-specific morphological and physiological traits, the human lineage also exhibited variations in stature and BMR, distinguishing anatomically modern humans (AMHs) from other vertebrates and our closest evolutionary relatives, the great apes.5,6,7 AMHs have increased stature and an elevated metabolism level compared to our closest living relatives; AMHs possess a stature ∼0.3 m higher8 and a BMR ∼15%–50% higher, adjusted for body mass, than that of non-human great apes (including chimpanzees, bonobos, and orangutans).9
It has been posited that a dietary shift from herbivory to carnivory may have played a pivotal role in the evolution of traits in AMHs, including increased height and BMR, alongside other notable characteristics, such as the development of larger, more complex brains; bipedalism; an extended lifespan; reduced gut length; smaller yet sharper teeth; and augmented social cohesion.10,11,12 This hypothesis stems from the recognition that meat-enriched diets offer a concentrated source of nutrients, including all essential amino acids (EAAs), bioavailable iron and zinc, and essential vitamins.13 Additionally, meat consumption provides omega-3 fatty acids, which are vital for brain development, and fat stores that boast more than double the energy density per gram compared to plant-based food.13
While genome-wide association studies (GWASs) have extensively explored the genetic basis of height14,15,16 and BMR,17 particularly height, these investigations have largely neglected the evolutionary perspective. Furthermore, height growth, especially during developmental periods, requires significant energy to support bone elongation, tissue synthesis, and overall somatic maintenance.18 As BMR reflects the absolute whole-body baseline energy expenditure, the level of BMR indicates the status of energy metabolic homeostasis, which is likely a critical factor for bone growth and observed increases in height. However, the genetic underpinnings of increased height and BMR in AMHs remain elusive. It is unclear whether the observed increases in BMR and height in AMHs result from pleiotropic effects of human-specific mutations or from independent adaptive processes.
In this study, we explored the genetic basis shared between human height and BMR through a genome-wide association analysis of 458,303 UK Biobank participants. We identified 6,385 shared candidate causal variants, accounting for 19% and 29% of the candidate causal variants of height and BMR, respectively. A key finding was the identification of the human-specific mutation rs34590044-A, located in an enhancer region of acyl-coenzyme A (CoA) synthetase family member 3 (ACSF3), which emerged approximately 653,000 years ago. This mutation is associated with increased stature and elevated BMR in humans. Functional experiments confirmed that rs34590044-A enhances mitochondrial activity and reduces toxic metabolite accumulation, contributing to increased body length and energy expenditure, particularly in mice fed high-threonine diets, which are characteristic of meat-based diets. These findings suggest that the emergence of rs34590044-A may have played a role in the dietary and physiological evolution of AMHs, linking genetic and environmental influences in shaping key phenotypic traits such as height and BMR.
Zhang, Yufeng; Wang, Jie; Yi, Chuanyou; Su, Yue; Yin, Zi; Zhang, Shuxian; Jin, Li; Stoneking, Mark; Yang, Jian; Wang, Ke; Huang, He; Li, Jin; Fan, Shaohua
An ancient regulatory variant of ACSF3 influences the coevolution of increased human height and basal metabolic rate via metabolic homeostasis
Cell Genomics (2025); DOI: 10.1016/j.xgen.2025.100855
Copyright: © 2025 The authors.
Published by Elsevier Inc. Open access.
Reprinted under a Creative Commons Attribution 4.0 International license (CC BY 4.0)
What makes this especially problematic for creationist claims is that the mutation’s benefits are amplified in the context of a high-protein, meat-rich diet, tying genetic change to environmental interaction—one of the fundamental principles of evolution. It suggests not just random mutation, but mutation filtered by selective pressures in a specific ecological niche. To maintain the notion that this is somehow "devolutionary," creationists would need to argue that increasing energy efficiency and physiological capacity constitutes a loss of information—an argument that quickly collapses under scrutiny.
Moreover, invoking a “perfect” original genome becomes increasingly absurd in light of such findings. If the original genome were truly flawless, why would natural selection favour a mutation that improves energy metabolism? And how could perfection permit a regulatory change to take hold and spread through a population? This discovery highlights a reality that creationist models consistently fail to accommodate: beneficial mutations not only occur, but they can also drive key innovations in evolutionary history.
In short, this is yet another empirical demonstration that mutation, variation, and selection work in concert to shape complex traits—exactly as evolutionary biology predicts, and exactly as creationist dogma denies.
All titles available in paperback, hardcover, ebook for Kindle and audio format.
Prices correct at time of publication. for current prices.