Study Reveals Details of Process Driving Evolution & Major Diseases | NYU Langone News
A recent study by researchers at NYU Langone Health and Ludwig-Maximilians-Universität München has shed new light on how certain genetic elements, known as "jumping genes," contribute to both human evolution and the development of disease [1.1].
Intelligent Design (ID) proponents, such as Discovery Institute fellow William A. Dembski, claim that any genetic information which is both complex and specific can only originate from an intelligent designer. Similarly, Michael J. Behe argues that any complex biological structure or process requiring all its parts to function could not have evolved gradually and therefore must have been deliberately designed.
These arguments rest on little more than the fallacies of argument from incredulity and the God-of-the-gaps. Worse still, they inevitably raise troubling theological implications: if such "complex specified information" leads to harmful outcomes—such as diseases, congenital disorders, or parasitism—then their supposed designer must be incompetent, indifferent, or malevolent. In the case at hand, the study focuses on a type of "jumping gene," or retrotransposon, which is known to cause genetic diseases including cancers.
Proponents of ID consistently sidestep these issues, as they conflict with their effort to portray the Bible as a scientifically accurate text describing a benevolent, human-centred creator.
Can you simplify this news item for a lay readership and explain why it is another problem for ID creationism, especially Dembski's 'complex specified information', with a glossary of the technical terms, please. Understanding the StudyThe new research has just been published as an open-access article in Science Advances and is summarised in a news release from New York University Langone Health. A lay-friendly summary and analysis, prepared by AI (ChatGPT-4o) is in the right-hand information panel.
The research focuses on a specific type of jumping gene called LINE-1 (Long Interspersed Nuclear Element-1), which comprises about 20% of the human genome. Unlike most genetic elements that remain stationary, LINE-1 can move around within our DNA, a process known as retrotransposition. This movement can lead to genetic diversity but also has the potential to disrupt normal gene function, contributing to diseases such as cancer and neurological disorders.
The study reveals that LINE-1 exploits a particular phase during cell division when the nucleus temporarily disassembles, allowing LINE-1 to insert copies of itself into new locations within the genome. This insertion process is facilitated by LINE-1 forming clusters, or condensates, with a protein it produces called ORF1p. These condensates enable LINE-1 to bind effectively to DNA, promoting its replication and integration into the genome [2.1].
Implications for Intelligent Design and 'Complex Specified Information'
The findings present challenges to the concept of Intelligent Design (ID), particularly William Dembski's notion of "Complex Specified Information" (CSI). CSI posits that certain biological structures are too complex and specifically arranged to have arisen through natural processes, implying the necessity of an intelligent designer[3.1].
However, the study demonstrates that natural mechanisms, such as the activity of LINE-1 elements, can lead to significant genetic changes over time. These changes can increase genetic complexity and introduce new functions without the need for an external designer. This evidence supports the idea that evolutionary processes, driven by natural genetic variation and selection, are sufficient to account for the complexity observed in biological systems.[2.1,4.1]
Glossary of Technical Terms
- LINE-1 (Long Interspersed Nuclear Element-1): A type of transposable element capable of copying and inserting itself into different genomic locations.
- Jumping Genes: Segments of DNA that can move to different positions within the genome, also known as transposable elements.
- Retrotransposition: The process by which genetic elements like LINE-1 are transcribed into RNA, then reverse-transcribed back into DNA and inserted into new genomic locations.
- Condensates: Clusters formed by molecules, such as proteins and RNA, which facilitate specific biological processes.
- ORF1p: A protein encoded by LINE-1 that assists in the formation of condensates, enabling LINE-1 to bind to DNA.
- Cell Division (Mitosis): The process by which a cell divides to produce two new cells, during which the nucleus temporarily disassembles.
- Complex Specified Information (CSI): A concept from Intelligent Design theory suggesting that certain complex and specifically arranged biological structures cannot arise through natural processes.
In summary, the study provides compelling evidence that natural genetic mechanisms, such as those involving LINE-1 elements, play a significant role in driving evolutionary change and complexity. These findings challenge the assertions of Intelligent Design proponents by illustrating how intricate biological features can emerge through natural processes.
One staggering fact to emerge from the study is that this gene, or scattered remnants of it, account for 20% of the human genome.
Study Reveals Details of Process Driving Evolution & Major Diseases
Viruses are known to use the genetic machinery of the human cells they invade to make copies of themselves. As part of the process, viruses leave behind remnants throughout the genetic material (genomes) of humans. The viruslike insertions, called “transposable elements,” are snippets of genetic material even simpler than viruses that also use host cell machinery to replicate.
Nearly all these inserted elements have been silenced by our cells’ defense mechanisms over time, but a few, nicknamed “jumping genes,” can still move around the human genome like viruses. Just one, long interspersed nuclear element 1 (LINE-1), can still move by itself.
As an element type that behaves like the retrovirus HIV, the LINE-1 “retrotransposon” is first copied into a molecule of RNA, the genetic material that partners with DNA, and then the RNA LINE-1 copy is converted back into DNA in a new place in the genome. In this way, retrotransposons add code to the human genome every time they move, which explains why 500,000 LINE-1 repeats now represent a “staggering” 20 percent of the human genome. These repeats drive genome evolution, but can also cause neurological diseases, cancer, and aging when LINE-1 randomly jumps into essential genes, or triggers an immune response like a virus to cause inflammation.
To copy itself, however, LINE-1 must enter each cell’s nucleus, the inner barrier that houses DNA. Now a new study, published online May 2 in the journal Science Advances, reveals that LINE-1 binds to cellular DNA during the brief periods when nuclei break open as cells continually divide in two, creating replacements to keep tissues viable as we age. The research team found that LINE-1 RNA takes advantage of these moments, assembling into clusters with one of the two proteins it encodes, ORF1p, to hold tightly to DNA until the nucleus reforms after cell division.
Led by researchers at NYU Langone Health and the Munich Gene Center at Ludwig-Maximilians-Universität (LMU) München in Germany, the work revealed specifically that LINE-1 can only bind to DNA when ORF1p—which can bind to RNA, DNA, and itself in linked copies called multimers—accumulates into clusters of hundreds of molecules called condensates. As more ORF1p molecules build up, they eventually envelop the LINE-1 RNA, which makes more binding sites available for the entire cluster to attach to DNA.
Our study provides crucial insight into how a genetic element that has come to make up a large part of human DNA can successfully invade the nucleus to copy itself. These findings on the precise mechanisms behind LINE-1 insertion lay the foundations for the design of future therapies to prevent LINE-1 replication.
Associate Professor Liam J. Holt, PhD, senior author
Department of Biochemistry and Molecular Pharmacology and the Institute for Systems Genetics
New York University Grossmann School of Medicine, New York, NY, USA.
The work also suggests that the LINE-1 condensate acts as a delivery vehicle to bring its RNA into proximity of the right sequences (rich in the DNA bases adenine and thymine) on DNA where the retrotransposon tends to insert, say the study authors. Packaged in its condensates, LINE-1 is thought to evade mechanisms that exclude large particles from the nucleus during mitosis as a cellular defense against viruses.LINE-1 condensates have a remarkable feature in that their DNA binding ability emerges only when the ratio of ORF1p copies to RNA is high enough in the condensates. Moving forward, we will be looking to see if other condensates undergo functional changes as the ratios between their components change.
Associate Professor Liam J. Holt, PhD.
Along with Dr. Holt, the first study authors were graduate student Farida Ettefa at NYU Grossman School of Medicine and its Institutes for Systems Genetics and Sarah Zernia of Gene Center Munich at Ludwig-Maximilians-Universität (LMU) München in Germany. Also study authors were Cas Koeman, Joëlle Deplazes-Lauber, Marvin Freitag, and co-senior author Johannes Stigler from Ludwig-Maximilians-Universität München. The study was supported by the LMU-NYU Research Cooperation Program.
Publication:
AbstractThis study provides compelling evidence that, by the logic of Intelligent Design (ID) creationism, the retrotransposon in question—responsible for diseases such as cancer and autoimmune conditions—contains what ID proponents call "complex specified information" and forms an irreducibly complex structure, functioning only when all components are present. Taken on ID creationism's own terms, this would constitute evidence for an intelligent designer intimately involved in the process.
Long interspersed nuclear element–1 (LINE-1) is an autonomous retrotransposon that makes up a substantial portion of the human genome, contributing to genetic diversity and genome evolution. LINE-1 encodes two proteins, ORF1p and ORF2p, both essential for successful retrotransposition. ORF2p has endonuclease and reverse transcription activity, while ORF1p binds RNA. Many copies of ORF1p assemble onto the LINE-1 RNA to form a ribonucleoprotein (RNP) condensate. However, the function of these condensates in the LINE-1 life cycle remains unclear. Using reconstitution assays on DNA curtains, we show that L1 RNP condensates gain DNA binding activity only when RNA is super-saturated with ORF1p. In cells, L1 RNP condensates bind to chromosomes during mitosis. Mutational analysis reveals that DNA binding is crucial for nuclear entry and LINE-1 retrotransposition activity. Thus, a key function of ORF1p is to form an RNP condensate that gains access to the genome through DNA binding upon nuclear envelope breakdown.
INTRODUCTION
Transposable elements are genetic parasites that can move within the genome (1). The long interspersed nuclear element–1 (LINE-1, L1) is the only autonomously active retrotransposon (2) in humans and accounts for about 17% of the human genome (3, 4). The amplification of L1 in the human genome is one of the main drivers for genetic diversity and human evolution (5–7), but insertion into essential genes can also have severe effects on the host organism, contributing to the development of diseases such as neurodegeneration and cancer (8, 9). Thus, it is of great interest to understand the mechanisms underlying retrotransposition.
L1 encodes two proteins, ORF1p and ORF2p (10), that drive a copy-and-paste retrotransposition process (Fig. 1A). While both proteins are required for retrotransposition of L1, the role of ORF1p in the L1 life cycle is poorly understood (11, 12). ORF2p is an endonuclease and reverse transcriptase that catalyzes the insertion of a new copy of L1 into the host genome (2, 13). ORF1p has a low-nanomolar affinity for RNA and has been proposed to be an RNA chaperone (14–16). Upon translation of the 6-kb L1-mRNA, a dynamic assembly of multiple ORF1p trimers envelops the RNA and a small number of ORF2 proteins in a ribonucleoprotein (RNP) condensate (12, 17, 18). This RNP formation is essential for transposon activity in vivo (19, 20), but its functional role remains unclear.
ORF1p is composed of an N-terminal intrinsically disordered region (IDR), followed by a long coiled-coil (CC) domain and a globular C-terminal domain (CTD) containing the RNA recognition motif (RRM; Fig. 1A) (21–24). IDRs often facilitate multivalent interactions by electrostatic, polar and hydrophobic interactions (25–27); the ORF1p IDR was shown to be required for condensate formation (19, 20). In addition, the charge and position of a basic patch within the IDR are crucial for condensate formation (19) and retrotransposition activity in cells (12, 19, 28, 29). The IDR contains two highly conserved phosphorylation sites that are also required for retrotransposition activity (30, 31).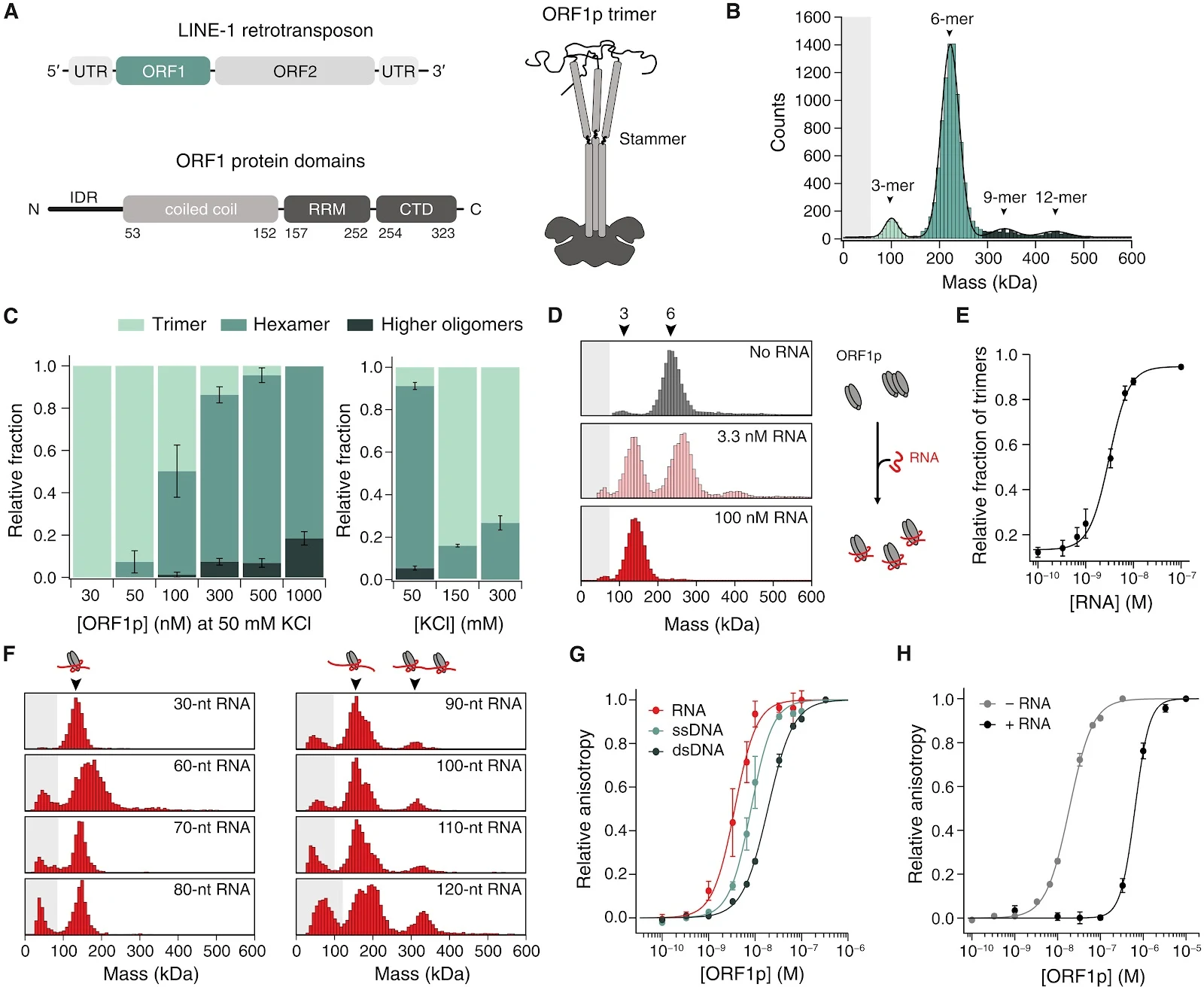 Fig. 1. ORF1p oligomerization is influenced by RNA.
Fig. 1. ORF1p oligomerization is influenced by RNA.
(A) Top: Schematic representation of L1 retrotransposon coding for ORF1p and ORF2p. Bottom: Domain composition of ORF1 protein containing an N-terminal IDR, a CC followed by an RRM and a CTD. Right: Schematic representation of the ORF1p homotrimer. (B) Mass photometry of 300 nM ORF1p at 50 mM KCl. The hexamer is the dominant species. Gray shading illustrates the detection limit. N = 4. (C) Left: Mass photometry of ORF1p concentration series at 50 mM KCl. With higher protein concentrations, more and larger oligomers are formed. Right: Mass photometry of KCl series at 300 nM ORF1p. Oligomerization is reduced at higher salt concentrations. Means ± SEM of N = 3. (D) Mass photometry of 300 nM ORF1p mixed with increasing concentrations of 30-nt RNA. RNA presence leads to a dissociation of the hexamer and RNP formation. N ≥ 4. (E) Relative fraction of trimers at increasing RNA concentrations fitted with a Hill equation. h = 2.1 ± 0.3, means ± SEM of N = 5. (F) Mass photometry of 300 nM ORF1p mixed with 50 nM RNA of different lengths. RNA with ≥90 nt provides sufficient space for two ORF1p trimers to bind. N = 3. (G) Fluorescence anisotropy of ORF1p binding to 1 nM 6-FAM-labeled 30-nt RNA, 30-nt ssDNA, or 30-bp dsDNA. The binding curve was fitted with a Hill equation, means ± SEM of N = 3. (H) Binding curves comparing binding of ORF1p to dsDNA [green, same as in (G)] versus ORF1p that was first saturated with 100 nM unlabeled 30-nt RNA and then bound to 1 nM 6-FAM–labeled 30-bp dsDNA (black). Prebound RNA reduced ORF1p’s affinity for dsDNA. Means ± SEM; −RNA: N = 3; +RNA: N = 4.
The ORF1p CC consists of 14 heptad repeats (32) that are interrupted by a three–amino acid stammer at positions 91 to 93 that has been proposed to increase CC flexibility (28, 33). Interaction of the CC domains facilitates formation of a dumbbell-shaped ORF1p homotrimer (11, 21, 23), which has previously been resolved by crystallography and nuclear magnetic resonance (NMR) spectroscopy in parts (28, 34, 35). ORF1p also assembles into higher oligomeric states (36, 37) of unclear structure; determining the nature of trimer-trimer interactions is crucial to resolve this question. We previously studied the processes of phase separation and condensation of ORF1p in vitro and in human cells, respectively, and established condensate formation as critical for L1 retrotransposition (19). However, the role of ORF1p oligomerization in L1 RNP assembly and retrotransposition remains unclear.
Oligomerization has been shown to affect ORF1p’s interactions with nucleic acids (36), which is of great interest given the requirement of L1 proteins to interact with both RNA and DNA during retrotransposition. Beyond its high affinity for RNA (36, 38), ORF1p has also been shown to bind to DNA in both bulk (36) and single-molecule experiments (37, 39). This raises the possibility that ORF1p could be involved in DNA target site recognition or priming. Previously, DNA binding activity was investigated with ORF1p alone, but never within assembled RNP complexes. As a result, it is unknown whether this DNA binding capability is restricted to the isolated protein or could be extended to the ORF1p-RNP and whether ORF1p has another role in retrotransposition besides RNA protection.
Here, we combine single-molecule DNA curtain (40) technology, mass photometry (41), and fluorescence anisotropy with high-resolution cell imaging to reveal the interplay between protein oligomerization and nucleic acid binding, RNP assembly and its localization to DNA, and L1 retrotransposition. We found that ORF1p forms multimers of trimers. Trimer multimerization is driven by electrostatic interactions and tuned by RNA binding. Furthermore, we generated RNP condensates by mixing L1 RNA and ORF1p and determined that these RNP condensates bind to DNA. By altering RNP composition, we show that excess ORF1p is required to generate double-stranded DNA (dsDNA) binding RNP condensates, consistent with the high stoichiometry of ORF1p observed in cells (12). Mutational studies suggest a connection between oligomerization behavior and stable DNA binding of RNP condensates. We present that L1 RNP condensates colocalize with mitotic chromatin in cells and can only gain access to the nucleus to successfully complete retrotransposition if they interact with DNA. Together, our study establishes direct DNA binding as an additional property of L1 RNP condensates. Thus, we provide insights into how ORF1p oligomerization and nucleic acid interactions are interdependent in RNP condensate formation and target site recognition and determine a crucial role for ORF1p-RNP condensates to initiate L1 retrotransposition.
In other words, following ID creationist reasoning, the study offers support for a malevolent designer—one apparently committed to increasing suffering through genetic mechanisms that randomly afflict human beings.
An explanation from an ID advocate as to why such features are only considered evidence of an omnibenevolent deity when they benefit humans, but not when they favour parasites or—as in this case—a parasitic segment of DNA that harms humans, is eagerly awaited.
All titles available in paperback, hardcover, ebook for Kindle and audio format.
Prices correct at time of publication. for current prices.











No comments:
Post a Comment
Obscene, threatening or obnoxious messages, preaching, abuse and spam will be removed, as will anything by known Internet trolls and stalkers, by known sock-puppet accounts and anything not connected with the post,
A claim made without evidence can be dismissed without evidence. Remember: your opinion is not an established fact unless corroborated.