
The problem with having a god who exists merely to fill gaps in human knowledge and understanding — as the god of creationism does — is that science has been steadily shrinking those gaps ever since the scientific method emerged and the Church lost its power to persecute scientists for discovering inconvenient truths. Today, only a few small gaps remain, scattered throughout the body of scientific knowledge —particularly in biology, which holds special interest for creationists.
Creationism persists because there are still people with such a poor understanding of science that they believe the authors of ancient religious texts — written during the Bronze Age, when humanity's knowledge gaps encompassed nearly everything in their small world — had access to some deeper, divine insight. Although what they wrote is often naively simplistic and demonstrably wrong in almost every respect, creationists insist that it somehow surpasses anything modern science has produced in terms of accuracy and reliability.
One of the few remaining gaps where creationists attempt to place their god — the abiogenesis gap — has just shrunk further. Predictably, this will be ignored, dismissed, or misrepresented by creationist frauds who exploit carefully maintained ignorance to preserve their cult followings and income streams.
This discovery by chemists at University College London and the Medical Research Council Laboratory of Molecular Biology reveals how a simple RNA molecule can self-replicate under conditions thought to have existed on prebiotic Earth. Many scientists believe this marks the origin of RNA-based life, which eventually gave rise to the more complex protein- and DNA-based life we see today. A self-replicating RNA molecule, competing for limited resources, will naturally evolve to become more efficient — leaving more copies of itself than rival variants. This is classic Darwinian evolution, operating in a context Darwin himself could scarcely have imagined, knowing nothing of RNA or DNA.
The new research is published open access in Nature Chemistry.
Abiogenesis: Progress on Life’s Origins. Abiogenesis is the process by which life arose naturally from non-living matter on early Earth. While the full picture is still emerging, science has made remarkable strides in recent decades. Several key theories and experimental advances have helped illuminate possible pathways:It is also explained in a UCL press release:
The RNA World Hypothesis
One of the most widely supported models suggests that RNA—a molecule capable of both storing genetic information and catalysing chemical reactions—was the first self-replicating molecule. The recent UCL discovery supports this, showing RNA molecules can self-replicate under realistic prebiotic conditions.
Prebiotic Chemistry
Experiments like the Miller-Urey experiment (1953) demonstrated that amino acids, the building blocks of proteins, can form spontaneously under conditions simulating early Earth’s atmosphere. Modern research has refined these models to include deep-sea hydrothermal vents, volcanic pools, and even space-borne organics delivered by comets.
Hydrothermal Vent Theory
This theory proposes that life began at alkaline hydrothermal vents on the ocean floor. These environments provide natural proton gradients and rich mineral surfaces — ideal conditions for concentrating molecules and driving the chemical reactions necessary for life.
Lipid World Hypothesis
Another complementary idea is that lipid molecules formed primitive cell-like vesicles that could enclose replicating molecules, enabling early forms of metabolism and compartmentalisation—key steps toward cellular life.
Systems Chemistry & Metabolism-First Models
Some models suggest metabolism could have arisen before genetic information. These theories explore self-sustaining reaction networks that might have laid the groundwork for more complex biochemical systems.
Abiogenesis is no longer a mystery relegated to guesswork or faith — it’s an active field of science, with each discovery bringing us closer to understanding how life began on Earth.
Chemists recreate how RNA might have reproduced for first time
Chemists at UCL and the MRC Laboratory of Molecular Biology have demonstrated how RNA (ribonucleic acid) might have replicated itself on early Earth – a key process in the origin of life.
Scientists believe that, in the earliest life forms, genetic material would have been carried and replicated by strands of RNA, before DNA and proteins later emerged and took over.
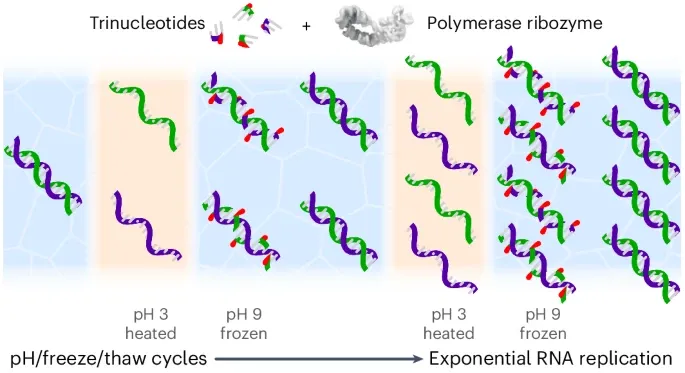
Yet getting strands of RNA to replicate in the lab in a simple way – i.e., that plausibly could have occurred at the outset of life – has proved challenging. RNA strands zip up into a double helix that blocks their replication. Like Velcro, these are hard to pull apart and quick to stick back together, leaving no time to copy them.
In a study described in Nature Chemistry, researchers overcame this problem by using three-letter “triplet” RNA building blocks in water and adding acid and heat, which separated the double helix. They then neutralised and froze the solution. In liquid gaps between the ice crystals, they saw that the triplet building blocks coated the RNA strands and stopped them from zipping back together – allowing replication to happen.
By thawing and beginning the cycle again, repeated changes in pH and temperature – which could plausibly occur in nature – allowed the RNA to replicate over and over again, with strands of RNA long enough to have a biological function and play a role in the origin of life.
Replication is fundamental to biology. In one sense, it is why we are here. But there’s no trace in biology of the first replicator. Even the single-celled organism that is the ancestor of all known life, the Last Universal Common Ancestor (LUCA), is a pretty complex entity, and behind it lies a lot of evolutionary history that is hidden from us.
Our best guess is that early life was run by RNA molecules. But a big problem for this hypothesis is that we haven’t been able to get a molecule of RNA to replicate itself in a way that could have occurred before life began several billion years ago. We can’t rely on a complex enzyme to do this, as happens in biology today. It needs to be a much simpler solution. The changing conditions we engineered can occur naturally, for instance with night and day cycles of temperature, or in geothermal environments where hot rocks meet a cold atmosphere.
The triplet or three-letter building blocks of RNA we used, called trinucleotides, do not occur in biology today, but they allow for much easier replication. The earliest forms of life are likely to have been quite different from any life that we know about. The models of biological species we are trying to build need to be simple enough to have emerged from the chemistry of early Earth.Dr James Attwater, lead author
MRC Laboratory of Molecular Biology
Cambridge Biomedical Campus, Cambridge, UK
and UCL Department of Chemistry, London, UK.
While the paper focuses solely on the chemistry, the research team said the conditions they created could plausibly mimic those in freshwater ponds or lakes, especially in geothermal environments where heat from inside the Earth has reached the surface.
However, this replication of RNA could not occur in freezing and thawing saltwater, as the presence of salt interferes with the freezing process and prevents RNA building blocks from reaching the concentration required to replicate RNA strands.
While a high concentration of RNA can also occur through evaporation, for instance a puddle evaporating in hot temperatures, RNA molecules are unstable at higher temperatures and more likely to break down, the researchers said.
Life is separated from pure chemistry by information, a molecular memory encoded in the genetic material that is transmitted from one generation to the next. For this process to occur, the information must be copied, i.e. replicated, to be passed on.
Dr Philipp Holliger, senior author
MRC Laboratory of Molecular Biology
Cambridge Biomedical Campus, Cambridge, UK.
The origin of life does not likely lie with RNA alone, but is thought to have emerged out of a combination of RNA, peptides (short chains of amino acids that are the building blocks of proteins), enzymes, and barrier-forming lipids that can protect these ingredients from their environment.
Several researchers at UCL are uncovering clues about how life began. In recent years, a team led by Professor Matthew Powner (UCL Chemistry) has demonstrated how chemistry could create several of the key molecules of life’s origin, including nucleotides (the building blocks of RNA and DNA) and amino acids and peptides (the building blocks of proteins), from simple molecular building blocks likely abundant on the early Earth.
Publication:
Abstract
RNA replication is considered a key process in the origins of life. However, both enzymatic and non-enzymatic RNA replication cycles are impeded by the ‘strand separation problem’, a form of product inhibition arising from the extraordinary stability of RNA duplexes and their rapid reannealing kinetics. Here we show that RNA trinucleotide triphosphates can overcome this problem by binding to and kinetically trapping dissociated RNA strands in a single-stranded form, while simultaneously serving as substrates for replication by an RNA polymerase ribozyme. When combined with coupled pH and freeze–thaw cycles, this enabled exponential replication of both (+) and (−) strands of double-stranded RNAs, including a fragment of the ribozyme itself. Subjecting random RNA sequence pools to open-ended replication yielded either defined replicating RNA sequences or the gradual emergence of diverse sequence pools. The latter derived from partial ribozyme self-replication alongside generation of new RNA sequences, and their composition drifted towards hypothesized primordial codons. These results unlock broader opportunities to model primordial RNA replication.
Main
Life on Earth relies on the faithful copying of its genetic material—replication—to enable heredity and evolution. This process is thought to have begun with the templated polymerization of activated mono- or oligonucleotide building blocks by chemical replication processes1,2,3 and later by RNA-catalysed RNA replication4,5,6,7. In its simplest form, RNA replication comprises the copying of (+) and (−) strands into complementary (−) and (+) daughter strands. For replication to proceed further, the double-stranded RNA replication products (duplexes) must again be dissociated into single-stranded RNAs, and these must be copied before they reanneal (Fig. 1a).
Fig. 1: Triplet substrates alleviate strand reannealing during RNA replication. a, The strand separation problem: the high energetic barrier of strand separation and speed of strand reannealing jointly inhibit RNA replication cycles. b, RNA strand copying by polymerization of trinucleotide triphosphates (triplets) upon a RNA template, catalysed by a TPR (a polymerase ribozyme using trinucleotide triphosphates as substrates, structure from ref. 21). Below, substrates for synthesis of the AD RNA duplex. Individual strands (A+ and A−) are shown hybridized to their complementary primers and triplets. c, TPR-catalysed RNA polymerization using 0.1 µM AD duplex or individual strands (A+, A−) as templates, showing product A− (top, fluorescein channel) and A+ (bottom, Cy5 channel). ‘AD acidified’ was pre-incubated in 2.5 mM HCl, and neutralized before reactions were frozen to initiate RNA polymerization (−7 °C for 48 h). Observed percentages of primer extended by >1 triplet, or reaching full length, are given after subtraction of levels in no-template controls (/). d, Effect of delaying ribozyme and triplet/primer addition after neutralization of the acidified AD template upon the percent of primers extended. Curve fitting indicates that AD reanneals with a t1/2 of 0.7 µM−1 min−1 (black circles, n = 3). Addition of triplets immediately upon AD neutralization (red squares, n = 3) essentially abolishes strand reannealing. ND, not detected. e, Revised scheme of an RNA replication cycle driven by triplet substrate inhibition of strand reannealing.
a, The strand separation problem: the high energetic barrier of strand separation and speed of strand reannealing jointly inhibit RNA replication cycles. b, RNA strand copying by polymerization of trinucleotide triphosphates (triplets) upon a RNA template, catalysed by a TPR (a polymerase ribozyme using trinucleotide triphosphates as substrates, structure from ref. 21). Below, substrates for synthesis of the AD RNA duplex. Individual strands (A+ and A−) are shown hybridized to their complementary primers and triplets. c, TPR-catalysed RNA polymerization using 0.1 µM AD duplex or individual strands (A+, A−) as templates, showing product A− (top, fluorescein channel) and A+ (bottom, Cy5 channel). ‘AD acidified’ was pre-incubated in 2.5 mM HCl, and neutralized before reactions were frozen to initiate RNA polymerization (−7 °C for 48 h). Observed percentages of primer extended by >1 triplet, or reaching full length, are given after subtraction of levels in no-template controls (/). d, Effect of delaying ribozyme and triplet/primer addition after neutralization of the acidified AD template upon the percent of primers extended. Curve fitting indicates that AD reanneals with a t1/2 of 0.7 µM−1 min−1 (black circles, n = 3). Addition of triplets immediately upon AD neutralization (red squares, n = 3) essentially abolishes strand reannealing. ND, not detected. e, Revised scheme of an RNA replication cycle driven by triplet substrate inhibition of strand reannealing.
Source data
However, RNA duplexes of functional lengths and concentrations (for example >25 nucleotides (nt), 100 nM) behave as essentially inert, ‘dead-end’ products due to their remarkable stability (with melting temperatures approaching the boiling point of water)8 . Furthermore, even when dissociated into individual, single-stranded RNAs, such strands reanneal on timescales (seconds to minutes) that are shorter than the typical time needed for copying reactions (hours to days) either by non-enzymatic processes or by polymerase ribozymes1. Thus, RNA replication cycles under standard conditions are both kinetically and thermodynamically disfavoured (Fig. 1a).
This so-called ‘strand separation problem’1 is aggravated by the comparative chemical instability of RNA. This precludes duplex dissociation under harsh conditions. High temperatures degrade RNA templates and ribozyme catalysts, particularly in the presence of divalent cations such as Mg2+ (which boost ribozyme activity but accelerate RNA fragmentation by transesterification)9. Furthermore, the strand separation problem worsens with increasing lengths of RNA duplexes, which become progressively harder to dissociate, more vulnerable to degradation and more prone to reannealing. A range of different approaches have been tried to overcome this fundamental barrier to open-ended RNA replication. Acidic pH can protonate the N1 of adenine and N3 of cytosine, disrupting base-pairing and destabilizing RNA duplexes10. Coupled with wet/dry cycles or ionic gradients in a thermophoretic setting, this has been shown to promote duplex melting and RNA assembly, and enable nucleic acid amplification by proteinaceous enzymes11,12,13,14. Furthermore, highly viscous solvents can slow RNA reannealing sufficiently for long (32 nt) substrates to be ligated15,16. Alternatively, strand-displacement syntheses can circumvent full duplex dissociation by the progressive addition of ‘invader’ oligonucleotides complementary to the non-templating strand17, or by the buildup of conformational strain on circular RNA templates18. Nevertheless, the scope of RNA-catalysed RNA replication cycles has been limited to polymerization of mononucleotides on primers flanking a 4-nt region assisted by denaturants19, or the templated ligation of up to three polynucleotide substrate segments14,20. However, general RNA replication and open-ended evolution requires the replication of longer sequences (able to encode a phenotype) via the polymerization of building blocks short enough to allow free sequence variation. In this Article we describe an approach that unlocks both the replication of longer RNA sequences and enables free sequence variation in replicating RNA pools. Our approach leverages an unexpected capacity of trinucleotide triphosphate (triplet) substrates to stabilize dissociated RNA strands. This can be coupled to cycles of pH, temperature and concentration to drive open-ended RNA replication by a polymerase ribozyme that utilises triplet substrates.
Attwater, J., Augustin, T.L., Curran, J.F. et al.
Trinucleotide substrates under pH–freeze–thaw cycles enable open-ended exponential RNA replication by a polymerase ribozyme. Nat. Chem. (2025). https://doi.org/10.1038/s41557-025-01830-y
Copyright: © 2025 The authors.
Published by Springer Nature Ltd. Open access.
Reprinted under a Creative Commons Attribution 4.0 International license (CC BY 4.0)
This new discovery — showing that simple RNA molecules can self-replicate under conditions plausibly present on prebiotic Earth — adds yet another piece to the puzzle of life’s origins. More importantly, it narrows one of the few remaining scientific gaps that creationists often point to as evidence of divine intervention: the origin of life itself, or abiogenesis. For decades, the inability of science to explain how life could begin without a designer has been a central plank in the creationist narrative. Each credible advance in this field chips away at that claim, replacing the unknown with natural, testable processes.
Creationism thrives on gaps — places where science has not yet offered a complete explanation — and then fills those gaps with supernatural assertions. The problem, of course, is that these gaps have been shrinking steadily for centuries. Where once gods were credited with thunder, disease, and celestial motion, science has provided natural explanations. Abiogenesis has long been among the last redoubts, but progress like this demonstrates that even here, nature is capable of extraordinary complexity without invoking supernatural causes.
Furthermore, this research shows that evolutionary mechanisms — variation, selection, and replication — may have been at work even before life as we define it began. This undermines the notion that life had to be a sudden, inexplicable event. Instead, it supports the view that life arose gradually from simpler chemical systems, governed by the same natural laws that apply elsewhere. Creationism isn’t just being challenged by biology — it’s being outflanked by chemistry and physics too.
In essence, every scientific breakthrough that explains how life could have arisen naturally is one less reason to assume it must have had a supernatural origin. Creationism relies on mystery to stay afloat, but discoveries like this make its pool of mystery ever more shallow.
All titles available in paperback, hardcover, ebook for Kindle and audio format.
Prices correct at time of publication. for current prices.










No comments:
Post a Comment
Obscene, threatening or obnoxious messages, preaching, abuse and spam will be removed, as will anything by known Internet trolls and stalkers, by known sock-puppet accounts and anything not connected with the post,
A claim made without evidence can be dismissed without evidence. Remember: your opinion is not an established fact unless corroborated.