Minute witnesses from the primordial sea | ETH Zurich
Scientists at Eidgenössische Technische Hochschule (ETH) Zürich have discovered evidence that may force us to rethink how Ice Ages and complex life began. By studying tiny iron oxide grains called ooides, they found that dissolved carbon dioxide in the oceans between 1000 and 541 million years ago was 90–99 percent lower than today — far too low to have triggered an Ice Age or the rise of multicellular organisms.
For creationists, this creates a dilemma. Whatever did cause these events, it happened hundreds of millions of years earlier than their literal reading of the Bible allows. Science may revise its conclusions when new evidence emerges, but it is nowhere near as wrong as the Bible’s authors—who missed the truth by billions of years.
This difference highlights the contrast between science and religion. Science asks, “What does the evidence show?” and adapts accordingly. Religion demands blind faith, treating doubt as a weakness.
Creationists boast that their “truth” never changes, but the reality is that what doesn't change is the mind of a creationist who is immune to evidence, deductive logic and analytical reasoning. Refusing to change your mind in the light of evidence is intellectual bankruptcy - the hallmark of dogma, not wisdom.
What are ooids? Ooids are tiny, spherical to egg-shaped grains, usually less than 2 mm across, formed of concentric layers of calcium carbonate or, less commonly, iron oxide around a nucleus such as a sand grain, shell fragment, or mineral particle. Their name comes from the Greek ōion, meaning “egg.”The findings are published in an open-access paper in Nature, and summarised in an ETH Zürich news release by Stéphanie Hegelbach.
How do they form?
- Shallow marine settings: Ooids typically form in warm, shallow, wave-agitated waters such as tropical shelves and lagoons, where constant motion keeps the particles rolling.
- Supersaturation: The water must be supersaturated with calcium carbonate (or iron in the case of ooides like those studied by ETH Zürich).
- Coating process: As the grain tumbles, minerals precipitate out of the water, forming successive concentric layers like the rings of an onion.
- Microbial influence: Modern research suggests microbial films on the particles may help nucleate and regulate mineral deposition.
Geological importance
- Environmental indicators: Ooids record chemical conditions of the seawater in which they grew, such as salinity, temperature, and carbon dioxide levels.
- Time markers: Because they only form under specific conditions, ancient ooid deposits can tell geologists a great deal about past climates and ocean chemistry.
- Fossil record: Ooid-rich limestones (called oolitic limestones) are common in the geological record, from the Precambrian to the present day. Famous examples include the “Bath Stone” used in English architecture.
Minute witnesses from the primordial sea
Researchers at ETH Zurich have been able to measure - for the first time - how the amount of dissolved organic carbon in the sea has changed over geological time. The results reveal that our explanations of how the ice ages and complex life forms came about are incomplete.
In briefEarth scientists often face huge challenges when researching the earth’s history: many significant events occurred such a long time ago that there is little direct evidence available. Consequently, researchers often have to rely on indirect clues or on computer models. The team led by ETH Professor Jordon Hemingway, however, has now discovered a unique natural witness to this period: tiny egg-shaped iron oxide stones that can be used to directly measure the carbon reserves in the primordial ocean.
- ETH researchers have refuted the assumption that a huge store of dissolved organic carbon in the ocean was partly responsible for the ice ages and the emergence of complex life between 1,000 and 541 million years ago.
- Applying a new method for analysing iron oxide grains, they were able – for the first time - to directly determine the dissolved organic carbon content of the ocean at that time.
- The measurements show that the amount of dissolved organic carbon in the oceans at that time must have been 90 to 99 per cent less than today.
- These findings call for new explanations as to how ecological and biogeochemical evolution are related.
Viewed on the outside, they resemble grains of sand, but in terms of their formation, these so-called ooids are more like rolling snowballs: they grow by layers as they are pushed across the sea floor by the waves. In the process, organic carbon molecules adhere to them and become part of the crystal structure.
Examining these impurities, Hemingway's team has succeeded in retracing the supply of organic carbon in the sea - by up to 1.65 billion years. In the journal Nature, the researchers show that, between 1,000 and 541 million years ago, this store was considerably lower than previously assumed. These findings refute the common explanations of significant geochemical and biological events of that time and cast a new light on the history of the Earth.
The ocean as a reservoir of life’s building blocks
How does carbon get into the oceans? On the one hand, carbon dioxide (CO2) dissolves from the air into seawater and is transported to the depths by mixing processes and ocean currents, where it is retained for a long time. On the other hand, organic carbon is produced by photosynthetic organisms such as phytoplankton or certain bacteria. Using the energy of sunlight and CO2, these microscopic organisms produce organic carbon compounds themselves. When the organisms die, they slowly sink towards the sea floor as marine snow. If it reaches the sea floor without being eaten by organisms along the way, the carbon is stored in the sea floor for millions of years.But it is not only phytoplankton that provides a supply of carbon components. The building blocks of life are also reused: microorganisms decompose excrement and dead organisms, thereby releasing the building blocks again. These molecules form what is known as dissolved organic carbon, which drifts freely in the ocean: a huge reservoir of building blocks that contains 200 times more carbon than is actually ‘built into’ marine life.Carbon cycle in the ocean 1,000 to 541 million years ago and today.Graphic: S. Hegelbach and J. Kuster / ETH Zurich.
The oxygen revolution changed everything
Based on anomalies in oceanic sedimentary rocks, researchers assumed that this building block reservoir must have been particularly voluminous between 1,000 and 541 million years ago. For a long time, this assumption served as the foundation for explaining how ice ages and complex life emerged at the same time. The photosynthetic production of the building blocks of life is closely linked to the development of the atmosphere and more complex life forms. It was only through photosynthesis that oxygen began to accumulate in the atmosphere.
In two waves - referred to as the oxygen catastrophes - the oxygen content rose to its current level of 21 per cent. Both events were accompanied by extreme ice ages that covered the entire planet in glaciers. Nevertheless, life continued to tinker and potter with new inventions: during the first oxygen catastrophe 2.4 to 2.1 billion years ago, organisms developed a metabolism converting food into energy with the help of oxygen. This exceedingly efficient way of generating energy enabled the development of more complex life forms.
Carbon content much lower than assumed
Hemingway's team is tracking such connections between geochemical and biological developments. The researchers have developed a new method that allows them to directly determine the size of the marine building block store at that particular time, based on the carbon particles in ooids.
Our results contradict all previous assumptions.
Professor Jordon D. Hemingway, senior-author
Geological Institute
Department of Earth and Planetary Sciences
ETH Zurich
Zurich, Switzerland.
According to the measurements taken by the ETH researchers, between 1,000 to 541 million years ago, the ocean did not contain more, but actually 90 to 99 per cent less dissolved organic carbon than it does today. It was only after the second oxygen catastrophe that the values rose to the current level of 660 billion tonnes of carbon.We need new explanations for how ice ages, complex life and oxygen increase are related.
Nir Galili. lead author
Geological Institute
Department of Earth and Planetary Sciences
ETH Zurich
Zurich, Switzerland.
He explains the massive shrinkage of the carbon store with the emergence of larger organisms at that time: single-celled and early multicellular organisms sank faster after their death, thereby increasing marine snowfall.
However, the carbon particles were not recycled in the deeper layers of the ocean because there was very little oxygen there. They settled on the sea floor, causing the reservoir of dissolved organic carbon to decline sharply. It was only when oxygen accumulated in the deep sea that the carbon reservoir grew back to its current volume.
From the primordial ocean to the present day
Although the periods studied are long past, the research findings are significant for the future. They change our view of how life on earth and possibly also on exoplanets has developed. At the same time, they help us understand how the earth responds to disturbances, and humans are one such disturbance: the warming and pollution of the oceans caused by human activities are currently leading to a decline in marine oxygen levels. Consequently, it cannot be ruled out that the events described could repeat themselves in the distant future.
 They look like ordinary pebbles: egg-shaped iron oxide stones under an electron microscope.Image: Nir Galili / ETH Zurich.
They look like ordinary pebbles: egg-shaped iron oxide stones under an electron microscope.Image: Nir Galili / ETH Zurich.
Publication:
Abstract
Dissolved organic carbon (DOC) is the largest reduced carbon reservoir in modern oceans1,2. Its dynamics regulate marine communities and atmospheric CO2 levels3,4, whereas 13C compositions track ecosystem structure and autotrophic metabolism5. However, the geologic history of marine DOC remains largely unconstrained6,7, limiting our ability to mechanistically reconstruct coupled ecological and biogeochemical evolution. Here we develop and validate a direct proxy for past DOC signatures using co-precipitated organic carbon in iron ooids. We apply this to 26 marine iron ooid-containing formations deposited over the past 1,650 million years to generate a data-based reconstruction of marine DOC signals since the Palaeoproterozoic. Our predicted DOC concentrations were near modern levels in the Palaeoproterozoic, then decreased by 90−99% in the Neoproterozoic before sharply rising in the Cambrian. We interpret these dynamics to reflect three distinct states. The occurrence of mostly small, single-celled organisms combined with severely hypoxic deep oceans, followed by larger, more complex organisms and little change in ocean oxygenation and finally continued organism growth and a transition to fully oxygenated oceans8,9. Furthermore, modern DOC is 13C-enriched relative to the Proterozoic, possibly because of changing autotrophic carbon-isotope fractionation driven by biological innovation. Our findings reflect connections between the carbon cycle, ocean oxygenation and the evolution of complex life.
Main
Marine dissolved organic carbon (DOC) contains 660 × 1015 g of carbon (gC), roughly equal to the pre-industrial atmospheric CO2 reservoir1,2. DOC is mainly produced by (1) planktonic communities in sunlit surface waters, in which dissolved compounds are either exuded directly by photosynthetic autotrophs or released during ecosystem interactions—for example, primary production, ‘sloppy feeding’, viral lysis and microbial loop interactions10—and (2) solubilization of sinking particulate organic carbon (POC) in the ocean interior. Subsequent transformation of solubilized carbon into more recalcitrant DOC compounds constitutes the microbial carbon pump1,4,11,12,13. By contrast, DOC is mainly consumed by POC scavenging and heterotrophic respiration in the deep ocean with rates that depend on particle sinking speed, temperature, dissolved O2 content ([O2]) and DOC concentration ([DOC]) by dilution effects and uptake kinetics1,14. Estimates suggest that 30% of deep-ocean DOC forms by the microbial carbon pump over decadal timescales (so-called labile, semi-labile and semi-refractory DOC), whereas the remainder has been circulating subject to slow respiration with a residence time of about 30,000 years (that is, 30 ocean overturning cycles; so-called refractory to ultra-refractory DOC, which includes carboxyl-rich alicyclic molecules)13,15,16. Given the long residence time of this refractory component, small changes in consumption rates could drive large perturbations in DOC reservoir size—and thus atmospheric CO2 levels (pCO2)—over approximately 103–105 year timescales3,4.
Deeper in the history of Earth, DOC dynamics have been invoked to explain global glaciation (snowball Earth) events17, deep-ocean oxygenation and the radiation of marine eukaryotes6,18,19,20,21,22. Specifically, in the Neoproterozoic Era (1,000–540 Ma), ref. 6 proposed that slow particle sinking before the evolution of multicellular and biomineralizing organisms23, combined with an anoxic deep ocean9,24, prompted a stronger microbial loop, slower respiration at depth and accumulation of a long-lived, recalcitrant DOC reservoir that was about 102–103 times its modern size. Subsequent evolution of faster sinking eukaryotes would increase POC burial flux and thus atmospheric O2 levels, whereas colder climates would increase O2 solubility; both mechanisms would drive deep-ocean oxygenation and transient respiration of this large DOC reservoir6. Furthermore, DOC exhibits lower δ13C values (‰ Vienna Pee Dee Belemnite (VPDB); Methods) relative to dissolved inorganic carbon (DIC = CO2(aq) + H2CO3 + HCO3− + CO32−) because of photosynthetic carbon-isotope fractionation (εp ≈ δ13CDIC − δ13Corg, where ‘org’ refers to bulk organic carbon). Transient DOC respiration to CO2 would thus produce 13C-depleted DIC and could explain anomalously large negative isotope excursions observed in Neoproterozoic carbonate rocks (that is, because δ13Ccarb ≈ δ13CDIC), particularly if the amount of DOC oxidized is large relative to the DIC reservoir size6,18,20. According to this model, a large DOC reservoir is thus a fundamental requirement to explain the Neoproterozoic geologic record.
Still, other studies reject the large DOC reservoir hypothesis. For example, ref. 25 suggested that respiration of this reservoir would deplete the surface of Earth of terminal electron acceptors (O2, SO42−) on timescales much shorter than those of observed isotope excursions. Similarly, ref. 26 demonstrated that δ13Corg and δ13Ccarb are coupled in the latest Neoproterozoic, requiring respiration of an alternative carbon source such as methane (CH4)—which exhibits lower δ13C values than both DOC and DIC—as a driver of isotope excursions. Furthermore, some authors have proposed that negative isotope excursions—despite being globally distributed and broadly synchronous—do not reflect the general state of the global carbon cycle, but instead implicate mechanisms such as late-stage diagenesis27, authigenic carbonate precipitation28 or marine transgressions29. Consistent with these interpretations, a recent model proposed that decreased DOC respiration in the Proterozoic was balanced by decreased production via the microbial carbon pump in anoxic deep oceans, leading to largely invariant [DOC] through geologic time7.
Such disagreement on the geologic history of marine DOC results from a dearth of direct records. To address this, we developed a proxy based on the content and δ13C value of organic carbon co-precipitated with iron (oxyhydr)oxides (termed Fe-OC; here including amorphous ferrihydrite and crystalline goethite and haematite). We show that Fe-OC loadings and 13C compositions quantitatively track [DOC] and δ13CDOC. We then apply this proxy to geologically preserved marine iron ooids—sand-sized grains with concentric iron (oxyhydr)oxide laminae that form in well-constrained environments30—to generate one of the first data-based reconstructions of marine DOC signals since the late Palaeoproterozoic.
Fig. 1: Fe-OC spatial distribution within goethite ooids.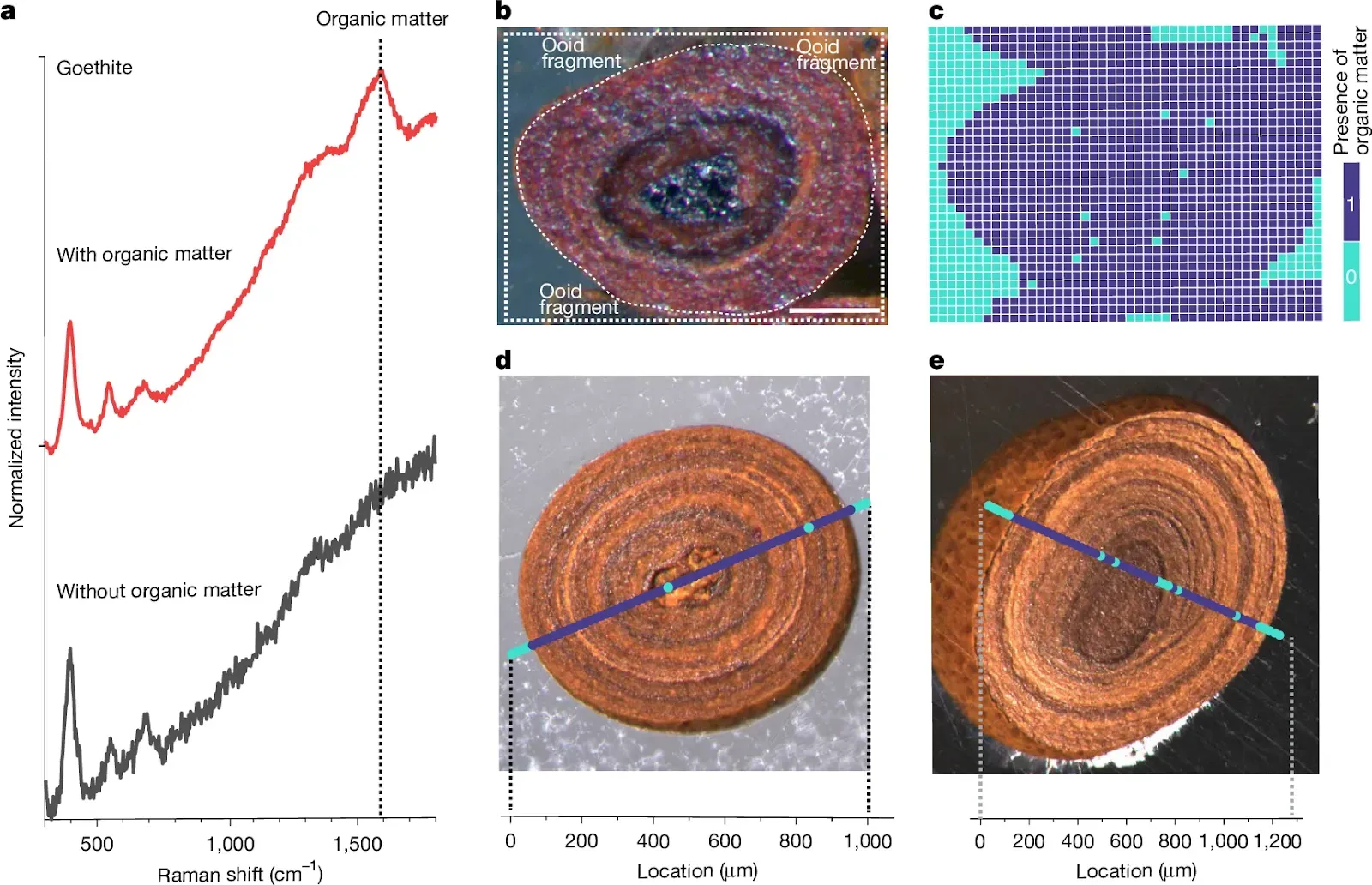 a, Raman spectra of synthetic goethite synthesized without organic matter (black line) and of a natural goethite ooid-containing organic matter (red line). The synthetic goethite was synthesized at 70 °C using FeCl3 as the Fe(III) source at pH 7 and aged for 60 days. The red spectrum is from a goethite ooid sample from the Aseri Formation (Ordovician, Estonia), the same as in d. A diagnostic peak at approximately 1,590 cm−1 (G-band) indicates the presence of organic carbon in goethite (Methods). b,c, An optical microscopy image and corresponding 2D Raman map of a modern ooid from Panarea Island, Italy, showing the presence (purple) and absence (turquoise) of Fe-OC at each pixel. d,e, Example line scans of goethite ooids from the Aseri Formation and the Sillaoru Formation (both Ordovician, Estonia), respectively, showing the spatial distribution of Fe-OC. The presence of OM was identified using the ratio I1,450–1,700/I1,200–1,450 (where I represents the integrated intensity within the defined spectral windows), and a threshold value determined from the raw data was used to convert calculated values to a binary format indicating the presence or absence of organic matter (Methods). Scale bar, 100 μm (b).Galili, N., Bernasconi, S.M., Nissan, A. et al.
a, Raman spectra of synthetic goethite synthesized without organic matter (black line) and of a natural goethite ooid-containing organic matter (red line). The synthetic goethite was synthesized at 70 °C using FeCl3 as the Fe(III) source at pH 7 and aged for 60 days. The red spectrum is from a goethite ooid sample from the Aseri Formation (Ordovician, Estonia), the same as in d. A diagnostic peak at approximately 1,590 cm−1 (G-band) indicates the presence of organic carbon in goethite (Methods). b,c, An optical microscopy image and corresponding 2D Raman map of a modern ooid from Panarea Island, Italy, showing the presence (purple) and absence (turquoise) of Fe-OC at each pixel. d,e, Example line scans of goethite ooids from the Aseri Formation and the Sillaoru Formation (both Ordovician, Estonia), respectively, showing the spatial distribution of Fe-OC. The presence of OM was identified using the ratio I1,450–1,700/I1,200–1,450 (where I represents the integrated intensity within the defined spectral windows), and a threshold value determined from the raw data was used to convert calculated values to a binary format indicating the presence or absence of organic matter (Methods). Scale bar, 100 μm (b).Galili, N., Bernasconi, S.M., Nissan, A. et al.
The geologic history of marine dissolved organic carbon from iron oxides. Nature 644, 945–951 (2025). https://doi.org/10.1038/s41586-025-09383-3
Copyright: © 2025 The authors.
Published by Springer Nature Ltd. Open access.
Reprinted under a Creative Commons Attribution 4.0 International license (CC BY 4.0)
What this new research highlights most clearly is the strength of science compared to dogma. Far from being a weakness, science’s willingness to revise its explanations when new data appear is its greatest strength. Knowledge grows, models are refined, and our understanding of Earth’s history becomes ever more detailed and accurate.
Creationism, by contrast, cannot grow. It is chained to an ancient text, written by people with no knowledge of geology, biology, or chemistry, and it demands loyalty to those errors regardless of the evidence. Where science welcomes questions and doubt as the engines of discovery, creationism fears them as threats to faith.
The ETH Zürich study does not overturn science—it enriches it. It reminds us that the story of life and climate on Earth is complex, unfolding over hundreds of millions of years under natural laws. Creationism, on the other hand, would have us believe that all of this was conjured in a few days by magic. The difference between the two approaches could not be starker: one seeks truth through evidence, the other clings to fiction in defiance of it.
Advertisement
What Makes You So Special? From The Big Bang To You
How did you come to be here, now? This books takes you from the Big Bang to the evolution of modern humans and the history of human cultures, showing that science is an adventure of discovery and a source of limitless wonder, giving us richer and more rewarding appreciation of the phenomenal privilege of merely being alive and able to begin to understand it all.
Ten Reasons To Lose Faith: And Why You Are Better Off Without It
This book explains why faith is a fallacy and serves no useful purpose other than providing an excuse for pretending to know things that are unknown. It also explains how losing faith liberates former sufferers from fear, delusion and the control of others, freeing them to see the world in a different light, to recognise the injustices that religions cause and to accept people for who they are, not which group they happened to be born in. A society based on atheist, Humanist principles would be a less divided, more inclusive, more peaceful society and one more appreciative of the one opportunity that life gives us to enjoy and wonder at the world we live in.
All titles available in paperback, hardcover, ebook for Kindle and audio format.
Prices correct at time of publication. for current prices.















No comments:
Post a Comment
Obscene, threatening or obnoxious messages, preaching, abuse and spam will be removed, as will anything by known Internet trolls and stalkers, by known sock-puppet accounts and anything not connected with the post,
A claim made without evidence can be dismissed without evidence. Remember: your opinion is not an established fact unless corroborated.