U of T researchers discover ‘trojan horse’ virus hiding in human parasite | Donnelly Centre for Cellular and Biomolecular Research The parasite, Toxoplasma gondii, is already notorious for manipulating the behaviour of its various hosts in ways which are highly detrimental to the host but highly beneficial to the parasite. For example, mice infected by it are positively attracted to felines - the primary host of the parasite - as a strategy for getting into the feline gut, where the sexual phase breeds and multiplies rapidly to produce oocytes which are excreted in the feline's faces, to be ingested by their secondary hosts, including humans. Chimpanzees, another secondary host, is manipulated by the parasite to crave the scent of leopard urine. The moods changes that infected Human experience may be a modified form of the chimpanzee behaviour, inherited from the common ancestor.
As though that wasn't embarrassing enough for devotees of the god whom they believe designs all living things, an international team of scientists led by researchers at the University of Toronto has found a new RNA virus that they believe is hitching a ride with Toxoplasma gondii which acts as a Trojan horse to gain the virus access to its hosts.
The difference being that, unlike the real Greeks who left what looked like a giant wooden effigy that the defenders thought was some sort of departing offering to their gods, so took into Troy willingly, only for the soldiers inside to come out at night, open the city gates and let the returning Greeks take the city, creationism's nasty little designer doesn't even make toxoplasma gondii look like an attractive gift, but sneaks that in too, in contaminated food and general environmental dirt and dust, that can get picked up on fingers.
Tell me all about the parasite Toxoplasma gondii, its life cycle and its effects on its human hosts, please. Toxoplasma gondii OverviewAn estimates 30% of the world human population are believed to have or have had, toxoplasmosis caused by T. gondii, risong to 100% in some areas. The USA figure is estimated at 25%.
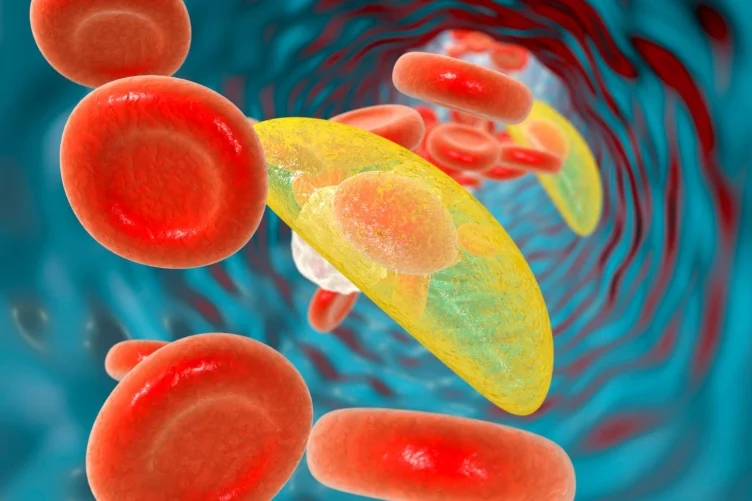
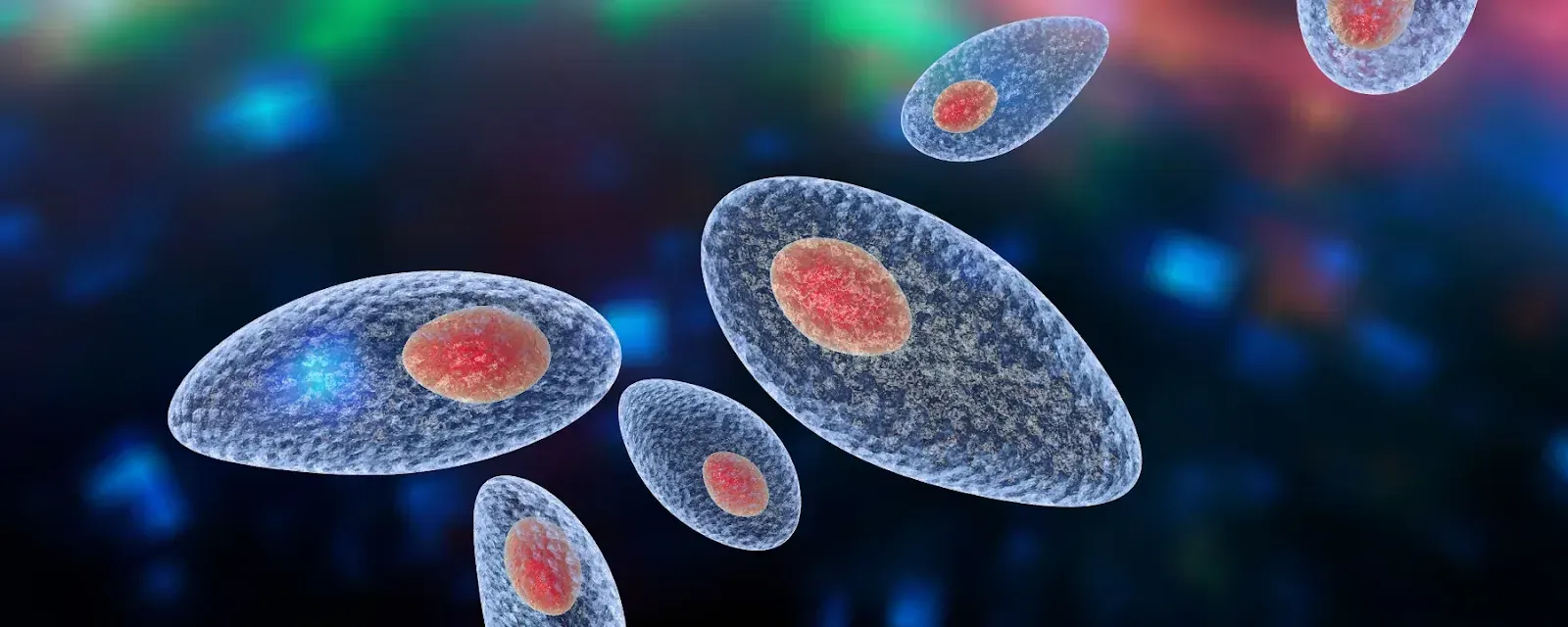
Toxoplasma gondii is a protozoan parasite that causes toxoplasmosis. It is an obligate intracellular parasite, meaning it must live and reproduce inside host cells. T. gondii is found worldwide and can infect most warm-blooded animals, including humans. Its primary host, where it completes its sexual reproduction, is the domestic cat and other felines.
Lifecycle of Toxoplasma gondii
Effects in Human Hosts
- Sexual Phase (Definitive Host - Felines):
- The parasite undergoes sexual reproduction in the intestines of felines.
- Oocysts are shed in the feces of the infected cat.
- These oocysts become infective within 1-5 days in the environment.
- Asexual Phase (Intermediate Hosts - Various Animals):
- Intermediate hosts (including humans) become infected by ingesting sporulated oocysts from contaminated soil, water, food, or surfaces.
- Oocysts release sporozoites in the intestines, which invade intestinal cells and differentiate into tachyzoites.
- Tachyzoites multiply rapidly and disseminate throughout the host's body, invading various tissues.
- Tachyzoites convert into bradyzoites and form tissue cysts in muscles and the brain, which are a chronic, latent form of the parasite.
- Human Infection:
- Humans can become infected through several routes:
- Ingesting undercooked or raw meat containing tissue cysts.
- Consuming food or water contaminated with oocysts.
- Vertical transmission from mother to fetus during pregnancy.
- Organ transplantation or blood transfusion (rare).
Diagnosis and Treatment
- Acute Toxoplasmosis:
- Most healthy individuals are asymptomatic or may experience mild flu-like symptoms (fever, swollen lymph nodes, muscle aches).
- In immunocompromised individuals (e.g., HIV/AIDS patients, organ transplant recipients), the infection can be severe, leading to encephalitis, pneumonitis, or chorioretinitis.
- Chronic Toxoplasmosis:
- Latent infection persists with tissue cysts, usually without symptoms.
- Some studies suggest a potential link between chronic infection and neurological or psychiatric disorders (e.g., schizophrenia, bipolar disorder), though this is still under investigation.
- Congenital Toxoplasmosis:
- If a woman acquires T. gondii infection during pregnancy, the parasite can cross the placenta and infect the fetus.
- The severity of congenital toxoplasmosis depends on the timing of infection:
- First trimester: Risk of severe outcomes, including miscarriage, stillbirth, or severe neurological damage.
- Later trimesters: Milder symptoms or asymptomatic at birth but potential for later development of vision or hearing problems, intellectual disabilities, or seizures.
Diagnosis:Treatment:
- Serological tests to detect specific antibodies (IgM and IgG).
- Molecular tests (PCR) to detect parasite DNA.
- Imaging studies (e.g., MRI, CT scans) in cases of suspected cerebral involvement.
Prevention
- Healthy individuals usually do not require treatment unless symptoms are severe or prolonged.
- For acute infection in immunocompromised individuals or pregnant women, treatment typically includes a combination of pyrimethamine and sulfadiazine, along with folinic acid to reduce bone marrow suppression.
- Congenital toxoplasmosis may require prolonged treatment with anti-parasitic medications.
Conclusion
- Properly cooking meat to safe temperatures.
- Washing hands and kitchen surfaces thoroughly after handling raw meat.
- Washing fruits and vegetables thoroughly.
- Avoiding consumption of untreated water.
- Pregnant women should avoid handling cat litter and take precautions if they must handle it (e.g., wearing gloves, washing hands thoroughly).
Toxoplasma gondii is a widespread parasite with a complex lifecycle involving both sexual and asexual reproduction. While it often causes mild or asymptomatic infections in healthy individuals, it can lead to severe complications in immunocompromised individuals and fetuses. Proper hygiene, food handling, and cooking practices are essential in preventing toxoplasmosis.
It also looks as though the two strains of T. gondii that carry the virus, Apocryptovirus odysseus, may be made especially virulent by it since they are the two most virulent strains known.
And that's not the only virus that humans can become infected with, using similar Trojan Horse tactics. There may be at least 18 others and possibly many more.
The discovery of this virus and its modus operandum is the subject of an open access research paper in the journal Virus Evolution. It is explained in a news release from the University of Toronto's Donnelly Centre and the Temerty Faculty of Medicine:
An international team led by researchers at the University of Toronto has found a new RNA virus that they believe is hitching a ride with a common human parasite.Technical detail is given in the team's paper in Virus Evolution:
The virus, called Apocryptovirus odysseus, along with 18 others that are closely related to it, was discovered through a computational screen of human neuron data – an effort aimed at elucidating the connection between RNA viruses and neuroinflammatory disease. The virus is associated with severe inflammation in humans infected with the parasite Toxoplasma gondii, leading the team to hypothesize that it exacerbates toxoplasmosis disease.
We discovered A. odysseus in human neurons using the open-science Serratus platform to search through more than 150,000 RNA viruses. Serratus identifies RNA viruses from public data by flagging an enzyme called RNA-dependent RNA polymerase, which facilitates replication of viral RNA. This enzyme allows the virus to reproduce itself and for the infection to spread.
Purav Gupta, first author
Donnelly Centre for Cellular and Biomolecular Research University of Toronto, Toronto, Canada.
The study was published recently in the journal Virus Evolution.
The parasite T. gondii is far-reaching, infecting an estimated one-third of the global population. It can live in any non-blood cell type, including neurons, forming cysts inside cells. The parasite is transmitted to nearby cells when the infected cell ruptures.
T. gondii infections often go unnoticed because they only lead to symptoms in rare cases. Regardless, toxoplasmosis merits investigation considering how widespread it is and the potential effects it may have on pregnant women and those who are immunocompromised, Gupta said.
We believe the virus and parasite work hand-in-hand to cause disease in the human host, where the virus hides inside the parasite, like a soldier in a trojan horse, to gain entry to the human brain. Our research marks the first time that scientists have connected toxoplasmosis to a virus.
Purav Gupta.
The newly discovered A. odysseus is found in two hypervirulent strains of the T. gondii parasite, referred to as RUB and COUGAR.
RUB has been documented in French Guinea to cause severe fever and organ failure, while COUGAR has been shown in British Columbia to be connected to ocular toxoplasmosis – the leading cause of infectious blindness. Researchers found the strains in different geographical locations at different times, demonstrating their potentially wide-ranging impacts.
Symptoms of toxoplasmosis can be aggravated by a hyperactivated human immune response. The virus-carrying parasite triggers this type of response when the immune system senses the foreign RNA of the virus.
The group of 19 RNA viruses we found are strong biomarkers for parasitic infection. It’s obvious now that the A. odysseus virus could be a valuable marker of disease-causing infections, like severe toxoplasmosis, in humans or other animals. The next step is to test if this raises the possibility that treating a parasite's viruses could be an effective means of treating symptoms that arise from parasitic infections.
This study underscores the importance of looking beyond the viruses that infect humans directly into the extended virome.
Assistant Professor Artem Babaian, corresponding author.
Assistant professor of molecular genetics
Donnelly Centre and the Temerty Faculty of Medicine.
University of Toronto, Toronto, Canada.
Zoonotic viruses that infect other living things in our environment in order to reach us are expected to cause the majority of emerging infectious diseases in humans, Babaian noted.
AbstractTo anticipate the usual creationist 'explanation' for these nasty little parasites by blaming them on another creator (Satan) using 'Sin' by some mysterious process that leaves creationism's god as the only entity capable of creating living organisms, while another creator is busy creating things over which it has no control. Or (from the slightly more theologically aware, but politically naive, creationists), blaming 'genetic entropy' and 'devolution' [sic], where, according the Michael J. Behe, in an abandonment of the pretense that creationism is not Christian fundamentalism in a grubby lab coat, 'Sin' following 'The Fall' causes the genome of a species to 'devolve' from an assumed perfection at 'creation'.
We are entering a ‘Platinum Age of Virus Discovery’, an era marked by exponential growth in the discovery of virus biodiversity, and driven by advances in metagenomics and computational analysis. In the ecosystem of a human (or any animal) there are more species of viruses than simply those directly infecting the animal cells. Viruses can infect all organisms constituting the microbiome, including bacteria, fungi, and unicellular parasites. Thus the complexity of possible interactions between host, microbe, and viruses is unfathomable. To understand this interaction network we must employ computationally assisted virology as a means of analyzing and interpreting the millions of available samples to make inferences about the ways in which viruses may intersect human health. From a computational viral screen of human neuronal datasets, we identified a novel narnavirus Apocryptovirus odysseus (Ao) which likely infects the neurotropic parasite Toxoplasma gondii. Previously, several parasitic protozoan viruses (PPVs) have been mechanistically established as triggers of host innate responses, and here we present in silico evidence that Ao is a plausible pro-inflammatory factor in human and mouse cells infected by T. gondii. T. gondii infects billions of people worldwide, yet the prognosis of toxoplasmosis disease is highly variable, and PPVs like Ao could function as a hitherto undescribed hypervirulence factor. In a broader screen of over 7.6 million samples, we explored phylogenetically proximal viruses to Ao and discovered nineteen Apocryptovirus species, all found in libraries annotated as vertebrate transcriptome or metatranscriptomes. While samples containing this genus of narnaviruses are derived from sheep, goat, bat, rabbit, chicken, and pigeon samples, the presence of virus is strongly predictive of parasitic Apicomplexa nucleic acid co-occurrence, supporting the fact that Apocryptovirus is a genus of parasite-infecting viruses. This is a computational proof-of-concept study in which we rapidly analyze millions of datasets from which we distilled a mechanistically, ecologically, and phylogenetically refined hypothesis. We predict that this highly diverged Ao RNA virus is biologically a T. gondii infection, and that Ao, and other viruses like it, will modulate this disease which afflicts billions worldwide.
Introduction
RNA virus discovery is undergoing a revolutionary expansion in the characterization of virus diversity (Shi et al. 2016, 2018; Wolf et al. 2020; Charon et al. 2022; Edgar et al. 2022.1; Neri et al. 2022.2; Zayed et al. 2022.3; Forgia et al. 2023; Hou et al. 2023.1; Lee et al. 2023.2; Olendraite, Brown, and Firth 2023.3; Zheludev et al. 2024). Of the predicted to virus species on Earth (Koonin et al. 2020.1), mammalian viruses are thought to have human-infecting potential (Anthony et al. 2013), of which we know ∼160 RNA viruses (Woolhouse and Adair 2013.1). This bulk of unknown zoonotic viruses (i.e., viruses that can transmit between non-human animals and humans) are expected to cause the majority of emerging infectious diseases in humans (Jones et al. 2008), with precedent set by the 1918 Spanish influenza, AIDS, SARS, Ebola, and more recently COVID-19. This establishes the real and continued threat that viral zoonoses pose to global health.
Most established relationships between a disease and its causal RNA virus are direct and proximal to infection and are thus tractable to reductionist interrogation. Yet it is evident that confounding variables can impede linking cause and effect: virus genetic heterogeneity, chronic infections, asymptomatic carriers, prolonged latency periods, and microbiome interactions all add complexity to the link between virus and disease. An indirect, yet causal, relationship is well exemplified by Epstein-Barr virus and multiple sclerosis (EBV-MS). The EBV-MS association has long been statistically postulated, yet clear evidence for causality was only established recently, on a background of increasing awareness of the role of neuroinflammation in neurodegeneration (Bjornevik et al. 2022.4; Lanz et al. 2022.5; Soldan and Lieberman 2023.4). Thus, statistical and computational inferences, while not sufficient to formalize causation, do allow for a radical simplification of the space of plausible hypotheses, and thereby accelerate the time to discovery. We opine that in addition to virus discovery, virus effect inference, pathological and ecological, should be a primary objective of the emerging field of computational virology.
Asking an old question in a new way The volume of freely available sequencing data in the Sequence Read Archive (SRA) has grown explosively for over a decade (Katz et al. 2021). Currently, there are in excess of 52.96 petabases (Pbp) of freely available sequencing data, capturing million biological samples. The emerging field of petabase-scale computational biology strives to analyze the totality of these data and enable expansive meta-analyses. The SRA-STAT project has processed over 10.8 million datasets (27.9 Pbp) using a k-mer hashing algorithm to create an index of reads matching known taxa genomes (Katz et al. 2021). Likewise, SERRATUS, which is aimed at uncovering known and novel RNA viruses using a translated nucleotide search for the RNA-dependent RNA polymerase (RdRp), the hallmark gene of RNA viruses, has processed over 7.5 million RNA sequencing datasets (18.97 Pbp) (Edgar et al. 2022.1). Our group focuses on leveraging this critical mass of freely available data to re-interrogate fundamental questions in virology using a data-driven philosophy. This approach allows us to minimize a priori bias and maximize the discovery of unexpected biology. Our immediate focus is to characterize highly divergent neuroinflammatory RNA viruses—these, we hypothesize, have the potential to cause or contribute to human neurological diseases of unknown etiology.
As simple as it gets—the Narnaviridae One group of poorly understood yet highly divergent viruses are the Narnaviridae. This clade of viruses are among the simplest viruses, comprising a naked, +ve sense RNA genome (hence the derivation from ‘naked RNA’) encoding an RNA-dependent RNA polymerase; the virus is thus observed as a ribonucleoprotein complex, with no true virion (Hillman and Esteban 2011; Hillman and Cai 2013.2). Members of this family of viruses are best known for their association with fungi, and indeed the first two species identified, Saccharomyces 20S RNA virus (ScNV-20S) and Saccharomyces 23S RNA virus (ScNV-23S), were discovered in the model organism Saccharomyces cerevisiae (Kadowaki and Halvorson 1971; Garvik and Haber 1978; Wejksnora and Haber 1978.1). ScNV-20S and ScNV-23S infections are mostly not associated with a phenotype (Hillman and Esteban 2011; Hillman and Cai 2013.2), although, as is the case with the S. cerevisiae L-A virus, chronic apathogenic infections may become phenotypic in specific genetic backgrounds (Chau et al. 2023.5). Likewise, although S. cerevisiae strains harboring high expression of a related narnavirus, N1199, display defects in sporulation, autophagy, and a change in metabolite utilization, strains with a low N1199 expression are more common and display no phenotype (Vijayraghavan et al. 2023.6). A comparison of virus-infected and virus-eliminated strains of Aspergillus flavus found that narnavirus infection is not associated with changes in colony appearance, growth rate, or mycelial/hyphal morphology, despite changes in transcriptomic profile (Kuroki et al. 2023.7). Besides fungi, Narnaviridae have also been found in marine protists (Charon, Murray, and Holmes 2021.1; Chiba et al. 2023.8), mosquitoes (Batson et al. 2021.2; Abbo et al. 2023.9; Yang et al. 2023.10), and other arthropods (Harvey et al. 2019; Xu et al. 2022.6).
Parasitic protozoan viruses, nested invaders The niches of the human virome extend beyond human cells; our holobiont constitutes an array of biological hosts including the bacteria, fungi, plants, and parasites. Of interest is the capacity of diverse viruses to modulate the physiology of these non-human hosts and in doing so, influence the biology of the ‘macrohost’—Homo sapiens (Gómez-Arreaza et al. 2017; Heeren et al. 2023.11; Zhao et al. 2023.12). Perhaps unsurprisingly, bacteria and their bacteriophages dominate the human microbiome and have been the focus of the majority of metagenomic research to date (Khan Mirzaei et al. 2021.3; Liang and Bushman 2021.4). Yet one intriguing category of human-adjacent viruses are parasitic protozoan viruses (PPVs) which infect the eukaryotic phyla Euglenozoa and Apicomplexa (Lye et al. 2016.1; Grybchuk et al. 2018.1; Charon et al. 2019.1; Rodrigues, Roy, and Sehgal 2022.7). PPVs are a diverse functional, rather than phylogenetic, grouping and are generally poorly characterized (Gómez-Arreaza et al. 2017; Heeren et al. 2023.11; Zhao et al. 2023.12). More than mere passengers, the presence of a PPV within a parasite and subsequent exposure of the macrohost to PPV-derived pathogen-associated molecular patterns (PAMPs) can modulate macrohost immune responses, with important implications for pathogenicity (Gómez-Arreaza et al. 2017; Zhao et al. 2023.12). Viral PAMPs, including viral RNA (vRNA), are sufficient to initiate an innate immune response via nucleic acid sensors (NAS), even in the context of parasitic infection of the macrohost. NAS include toll-like receptors (TLRs) which survey endosomes for dsRNA (TLR3), ssRNA (TLR7,8), or unmethylated CpG motifs in ssDNA (TLR9) (Fitzgerald and Kagan 2020.2). Additionally, the three RIG-I-like receptors (RLRs)—the signaling RIG-I and MDA5, and the regulatory LGP2—survey the cytosol for vRNA transcripts with an exposed 5ʹ triphosphate or misprocessed cellular RNA (Rehwinkel and Gack 2020.3). Collectively, NASs orchestrate an antiviral type I interferon (IFN) response (Lee and Ashkar 2018.2). For example, the dsRNA virus Cryptosporidium parvum virus 1 (CSpV1), which infects Cryptosporidium parvum, activates a type I IFN inflammatory cascade in mouse and cell culture models. Interestingly, this response undermines host defences against C. parvum, as evidenced by the enhanced antiparasitic immunity observed in mice lacking type I IFN receptor in their intestinal epithelia (Deng et al. 2023.13). While the underlying mechanism for the inflammation remains elusive, PPV presence appears to be necessary for parasite pathogenicity, potentially by diverting the host’s innate immune system toward the activation of antiviral immunity and away from antiparasitic immunity. The activation of a NAS-dependent type I IFN response by a PPV is also observed with Trichomonas vaginalis virus (TVV) infecting Trichomonas vaginalis and Leishmania RNA virus infecting Leishmania sp. (Fichorova et al. 2012; de Carvalho et al. 2019.2; Narayanasamy et al. 2022.8). In both of these common human pathogens, the virus is predicted or observed to worsen the severity of parasitic disease. Conversely, PPVs, such as Giardia lamblia virus 1 (GLV1), can also impair parasitic pathogenesis. In the case of GLV1, the virus inhibits the growth of G. lamblia (Miller, Wang, and Wang 1988), highlighting the complexity of this tripartite relationship. No such relationships, however, have been observed in narnavirus or narnavirus-like viruses.
Surprisingly, there are no known viruses associated with Toxoplasma gondii (T. gondii), an apicomplexan parasite that infects approximately 30 per cent of the global human population, with some geographic regions reaching majority seroprevalence (Pappas, Roussos, and Falagas 2009; Dubey 2021.5; Bisetegn et al. 2023.14). T. gondii has a notably broad host range of warm-blooded animals and tropism for all animal tissues, including the brain (Pappas, Roussos, and Falagas 2009). While T. gondii infection in Homo sapiens is most commonly asymptomatic and self-limiting, it remains a dangerous, opportunistic infection in immunocompromised patients and pregnant women, and a leading infectious cause of blindness (Khurana and Batra 2016.2; Goh et al. 2023.15). Furthermore, sporadic T. gondii strains are hypervirulent, with the ability to cause severe disease even in immunocompetent individuals (Dardé et al. 2020.4). Thus, the full global health burden of toxoplasmosis remains unclear, especially its possible role in chronic neuroinflammation and subsequent neurodegenerative disease.
Tell me, O Muse—a viral odyssey In this work, we use the SRA and SERRATUS to screen for potential human neuroinflammatory viruses, identifying Apocryptovirus odysseus, a narnavirus hidden within Toxoplasma gondii, and nineteen additional members of the proposed genus Apocryptovirus, all of which likely infect apicomplexan parasites. We establish these viruses in their phylogenetic context, estimate their prevalence, and perform sequence and structure analysis of the Apocryptovirus RdRp. Finally, we provide computational evidence and describe a model for Apocryptovirus odysseus-mediated Toxoplasma hypervirulence.
Purav Gupta, Aiden Hiller, Jawad Chowdhury, Declan Lim, Dillon Yee Lim, Jeroen P J Saeij, Artem Babaian, Felipe Rodriguez, Luke Pereira, Alejandro Morales-Tapia
A parasite odyssey: An RNA virus concealed in Toxoplasma gondii Virus Evolution, Volume 10, Issue 1, 2024, veae040, https://doi.org/10.1093/ve/veae040
Copyright: © 2024 The authors.
Published by Oxford University Press. Open access.
Reprinted under a Creative Commons Attribution 4.0 International license (CC BY 4.0)
The problem with that scientifically incoherent nonsense, is that a mutation which is advantageous can't logically be regarded as less perfect than what went before it, so to present this as 'devolution' involves a definition of advantageous that means 'worse than before', and requires some as yet unspecified mechanism for increasing 'devolved' alleles in the species gene pool, when all known environmental selectors tend to eliminate disadvantageous alleles and promote advantageous ones.
The ability of these viruses to hitch a ride on parasites and so gain access to new hosts, either evolved by Darwinian evolution, or, if you buy in to the creationist superstition, was deliberately created to do what it does. The scientifically nonsensical notions of 'genetic entropy' and 'devolution', stemming as they do straight from Christian fundamentalism and Bible literalism, can't rescue creationists from that dilemma.
The Malevolent Designer: Why Nature's God is Not Good
Illustrated by Catherine Webber-Hounslow.
The Unintelligent Designer: Refuting The Intelligent Design Hoax



Toxoplasmosis is one of the prime examples in Nature that the creator is a malevolent, cruel, sadistic monster. No way can this be the work of an all good being. No way no how. This is pure evil. It takes a devilish, diabolical mind to create this disgusting disease. Michael J. Be he is blind and delusional and doesn't realize that by crediting God with creating parasites and diseases, he is really insulting God. That's not praise and that's not flattering to say that God created such horrible things, Mr Michael J. Behe. This guy doesn't realize that his beliefs are really an insult and an indictment of God. Creating diseases and parasites is not something to be proud of. It's something to be ashamed of. Creationists are so delusional, blind and unable to think logically. They are afflicted with mental myopia.
ReplyDeleteThe list of embarrassments such as toxoplasmosis are literally infinite. The creator has an infinite number of cruelties to torture and kill His creation with. This illustrates what a lousy, horrible creator this is and certainly not a good one. It's not even a half way good being. If I had to put a ratio on the creator's goodness or lack of it, I would give it as 70 percent malevolent, and as 30 percent good, and this is being kind and generous a rating. The creator gets a failing grade. The world is screwed up because the creator is screwed up and the creator is screwed up because the world He made is screwed up, and much of His behavior in the Bible is screwed up. The Gnostics believe that the creator of this screwed up world is the demented Demiurge, also known as Yaldabaoth, or the blind idiot God. The Demiurge is a flawed, insane, demented, incompetent, amoral being with a Jekyll and Hyde personality. The true God is good and wise but weak and powerless. Gnostics believe in Dualism which implies God is weak, while Monism believes in a powerful, sovereign God who is cruel, insane, and stupid. Calvinism is the most extreme form of Monism, and its God is the cruelest of all.
Creationists are among the most delusional people in the world and cannot think logically.