Study shows western honey bee synthesizes food for its intestinal bacteria
A feature of creationism’s putative intelligent [sic] designer's designs is that they are almost always ingenious but overly complex for the task and often cobbled together from components designed for other purposes.
Just like a William Heath-Robinson contraption, where everyday objects such as a coal-scuttle full of coal, is a counterweight, every piece of string is made from short pieces of string knotted together and a simple task is made far more complicated than it need be.
And yet it works, just about, no matter how inefficiently, and removing just one component would make the whole thing fail - what creationists call 'irreducible complexity' but what a proper intelligent designer would probably call, 'bloody stupid'. Creationism's 'intelligent' [sic] designer wouldn't have lasted a term in design school.
Just such a Heath-Robinson machine has recently been discovered in the gut of the Western honey bee, Apis mellifera. Its purpose is to solve the problem of digesting the food the bee eats, for which it needs the help of 8 species of bacteria and other microorganisms that live in the bee's gut. Some of these bacteria are needed just to clean up the waste produced by other bacteria. 8 species is low compared to other species; humans, for example, need about 20 different species.
Apparently, giving bee's the same enzymes the bacteria use was far too simple and not nearly complex enough for the divine Heath-Robinson designer.
But even that wasn't complicated enough because one of the bacteria can't live on sugar alone, even though honey bees can, so the honey bee manufactures nutrients to feed to this bacteria.
How this was discovered was the subject of a recent paper in Nature Microbiology by a team of researchers led by Professor Philipp Engel in the University of Lausanne's Department of Fundamental Microbiology (DMF) in Dorigny. Their research is explained in a news release from the University of Lausanne (translated from French):
When bees nourish their microbiotaTechnical details of this Heath-Robinson contraption can be found in the team's open access paper in Nature Microbiology. Of course, being scientists, they explain it in terms of natural evolution, not the work of an invisible magician:
Two teams from UNIL and EPFL have succeeded in demonstrating that the insect synthesizes nutrients for native gut microbes. A study published in « Nature Microbiology ».
Bacteria have adapted to all terrestrial environments. Some have evolved to survive in the gut of animals, where they play an important role for their host; they provide energy by degrading indigestible food, they train and regulate the immune system, they protect against invasion by pathogenic bacteria, and they synthesize neuroactive molecules that regulate the behavior and cognition of their host.
These are great advantages for the host, but what advantages do the bacteria derive? Certainly, the host provides a comfortable home, but does the host also provide nutrients to native bacteria that enable them to colonize?
It is a difficult question that is possible to answer with the aide of … bees. Professor Philipp Engel in UNIL's Department of Fundamental Microbiology (DMF) in Dorigny has set his sights on the western honey bee (Apis mellifera). They are a relatively simple system to study compared to humans and their gut microbiota. Best known for the delicious honey they produce, this insect is also an excellent experimental model for gut microbiota research: it has acquired a remarkably simple and stable microbiota, composed of only around twenty bacterial species. In the laboratory of the Engel group, bees are raised without gut bacteria, and then fed specific species that will colonize the gut.
Full board for the bacteria
Bees love to gorge on nutrient rich pollen and honey, but they can also survive for long periods on a diet of only sugar water. But what happens to the gut bacteria? A study published on January 15, 2024 in Nature Microbiology by the Lausanne scientists reveals new insights: Dr. Andrew Quinn and PhD candidate Yassine El Chazli began by looking for evidence that the bacteria share nutrients with one another when bees receive nothing more than sugar water. Remember that intestinal bacteria are known to consume dietary nutrients as well as waste products from other microorganisms.
However, their first results left them perplexed: One specific bacterium in the gut, Snodgrassella alvi, cannot metabolize sugar to grow, and yet it still colonized the bee gut when sugar was the only food in the diet and no other bacteria were present.
By measuring metabolites in the gut, the scientists discovered that the bee synthesizes multiple acids (citric acid, malic acid, 3-hydroxy-3-methylglutaric acid, etc.) that are exported into the gut and were less abundant when S. alvi was present. These results led them to pose an unexpected hypothesis: Does the bee directly enable S. alvi to colonize its gut by furnishing the necessary nutrients?
Picture proof
Proving this hypothesis was surprisingly difficult, but fortunately, the key expertise was just across the road in the laboratory of Professor Anders Meibom (affiliated with UNIL and EPFL). Professor Meibom and his team are experts in measuring the flux of metabolites in complex environments at nanometers scale resolution by using one of the few NanoSIMS (Nanoscale Secondary Ion Mass Spectrometry) instruments in Europe.
Together the two teams devised an experiment in which microbiota free bees received a special diet of glucose where the natural 12C atoms of carbon in the glucose were replaced with the naturally rare 13C “labelled” isotopes. The bees were then colonized with S. alvi. At the end of the experiment, the fixed guts embarked on a journey, first passing by the electron microscopy facility of UNIL, led by Senior Lecturer Christel Genoud. Then, they moved on to the laboratory of professor Meibom and his NanoSIMS. In the end, the scientists were able to construct a 2-dimensional “image” of the 13C atoms in the gut of the bee, which showed that the S. alvi cells were significantly enriched in 13C, which reflected the 13C enrichment of the acids present in the gut.
To the rescue of the bees
Thus, in a single image, the team was able to show conclusively that the bee synthesizes food for its intestinal bacteria. “This is a wonderful example of cutting-edge, truly interdisciplinary scientific collaboration, which has brought together several scientific units within UNIL and EPFL," comments Anders Meibom. When we work together in this way, there are not many academic environments in the world that have more to offer," adds the professor, who is a pioneer in the application of NanoSIMS technologies to the intransigent questions of biology.
"It's possible that many other gut microorganisms also feed on host-derived compounds," says co-lead author Dr. Andrew Quinn, imagining an extension of this approach to other bacteria. Refocusing on bees: "These results could also explain why bees have such a specialized and conserved gut microbiota." And these mechanisms could play a role in bees' vulnerability to climate change, pesticides, or new pathogens: "Their vulnerability could result from a disruption in this intricate metabolic synergy between the bee and its gut microbiota. We already know that exposure to the herbicide glyphosate makes bees more susceptible to pathogens and reduces the abundance of S. alvi in the gut. Now, armed with these new findings, we're looking for answers to these pressing questions."
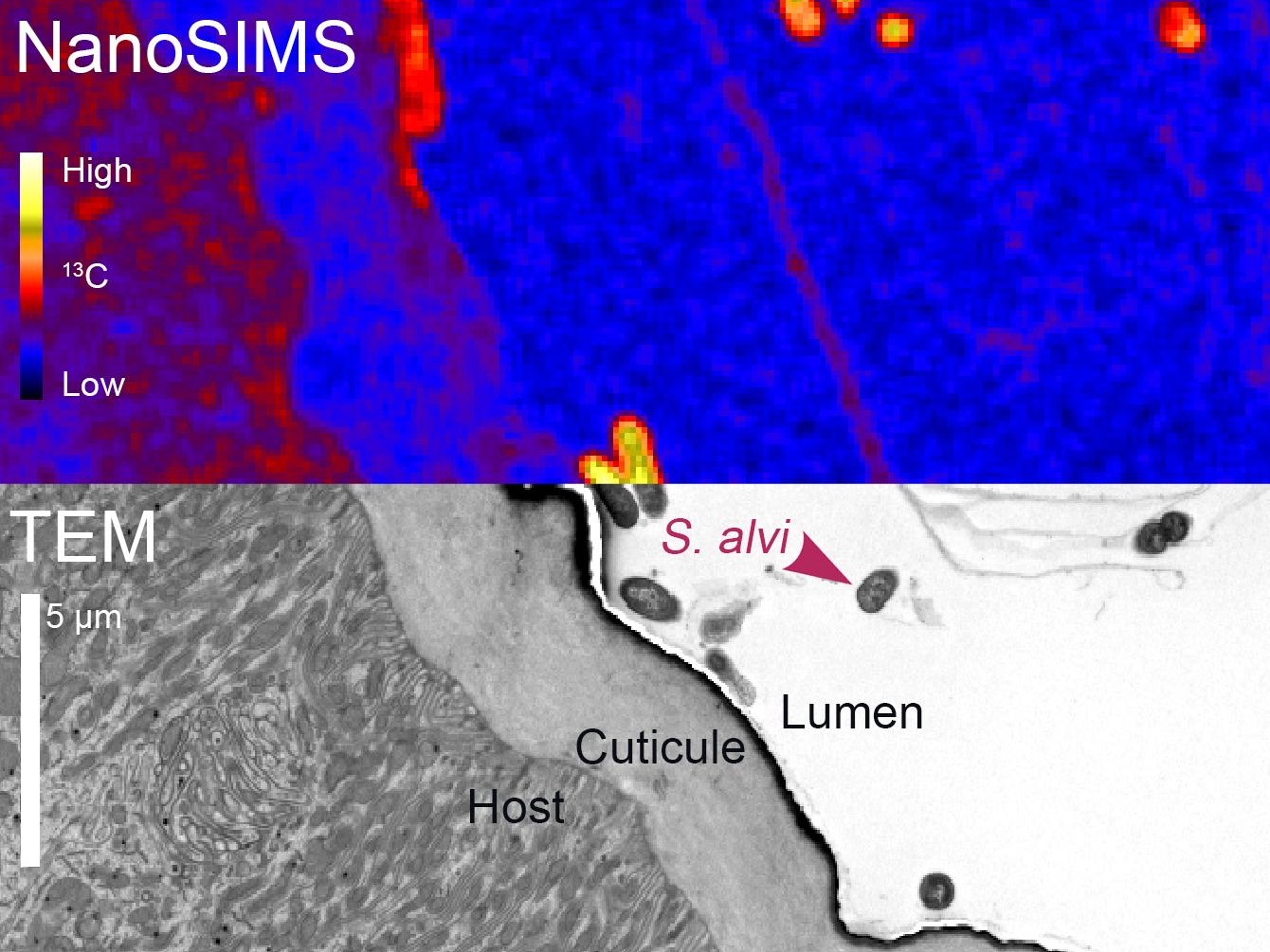 Visualization of the transfer of metabolites from the bee to its intestinal bacteria. Cross section of the ileum in the bee gut imaged by NanoSIMS (top) and transmission electron microscopy (bottom). The yellow and red colors of the oval bacterial cells show their incorporation of carbon-13 from metabolites produced by the host bee.
Visualization of the transfer of metabolites from the bee to its intestinal bacteria. Cross section of the ileum in the bee gut imaged by NanoSIMS (top) and transmission electron microscopy (bottom). The yellow and red colors of the oval bacterial cells show their incorporation of carbon-13 from metabolites produced by the host bee.
Abstract
Diverse bacteria can colonize the animal gut using dietary nutrients or by engaging in microbial crossfeeding interactions. Less is known about the role of host-derived nutrients in enabling gut bacterial colonization. Here we examined metabolic interactions within the evolutionary ancient symbiosis between the honey bee (Apis mellifera) and the core gut microbiota member Snodgrassella alvi. This betaproteobacterium is incapable of metabolizing saccharides, yet colonizes the honey bee gut in the presence of a sugar-only diet. Using comparative metabolomics, 13C-tracers and nanoscale secondary ion mass spectrometry (NanoSIMS), we show in vivo that S. alvi grows on host-derived organic acids, including citrate, glycerate and 3-hydroxy-3-methylglutarate, which are actively secreted by the host into the gut lumen. S. alvi also modulates tryptophan metabolism in the gut by converting kynurenine to anthranilate. These results suggest that S. alvi is adapted to a specific metabolic niche in the honey bee gut that depends on host-derived nutritional resources.
Main
Gut bacteria and their hosts typically engage in mutualistic interactions. Metabolic exchange from the gut microbiota to the host is vital for uptake of essential nutrients, gut health and immune system function1. In turn, bacteria profit from a stable niche environment and frequent supply of exogenous food. The role of host-secreted metabolites that benefit bacteria in the gut is less well understood. Such metabolic exchange is difficult to identify due to the overwhelming contributions of the diet and microbial products towards gut metabolites.
Deciphering the extent to which host metabolite secretion drives microbial colonization of native symbionts is aided by a simple, tractable system in which diet and microbiota-derived metabolites can be tightly controlled. The Western honey bee (Apis mellifera) provides such a system. Its gut microbiota is broadly stable and composed of only eight to ten genera2,3 many of which have longstanding evolutionary associations with their host that date back to the emergence of social bees >80 million years ago4,5,6. Bacteria of these genera are culturable and can be inoculated individually or as defined communities into gnotobiotic bees7. Furthermore, while the honey bee diet features a rich mixture of compounds found in nectar and pollen, bees can survive for extended periods on pure sugar water diets8.
Most members of the bee gut microbiome are primary fermenters, possessing a broad range of carbohydrate degradation enzymes that enable them to use hemicellulose, pectin, starch or glycosides found in pollen3,9. A notable exception is the Betaproteobacteria Snodgrassella alvi. It colonizes the cuticular surfaces of the ileum in the hindgut and displays a markedly different metabolism10,11. Lacking a functional glycolysis pathway, S. alvi profits from acids in the gut, generating energy from an aerobic tricarboxylic acid (TCA) cycle and biomass through gluconeogenesis. Fermentation by other microbial members has been proposed as the primary source of short chain fatty acids (SCFAs) consumed by S. alvi. In particular, bacteria of the gammaproteobacterial genus Gilliamella are probably mutualistic partners for metabolic crosstalk, as they colocalize with S. alvi within biofilms attached to the cuticular surface in the ileum and share complementary metabolic capabilities10. Experimental evidence from in vitro growth experiments bolstered this hypothesis, showing that S. alvi grows on the spent media of Gilliamella, while consuming numerous products of the Gilliamella metabolism, such as succinate and pyruvate12.
Although a strong case can be made for niche exploitation through bacterial crossfeeding, this does not explain previous results in which S. alvi was able to monocolonize bees fed diets of sugar water and pollen12. To better understand the nutrient sources that S. alvi exploits, we provided bees with a simple (sugar water) or complex (sugar water and pollen) diet and colonized them with S. alvi alone or together with divergent strains of the genus Gilliamella. Surprisingly, we found that a simple sugar water diet was sufficient for S. alvi to colonize the honey bee gut. Subsequent metabolomics analysis indicated that host-derived carboxylic acids enable S. alvi colonization. We validated this with a series of experiments showing that (1) these carboxylic acids are synthesized by the host, (2) S. alvi uses them for growth and (3) the findings hold across a range of divergent Snodgrassella strains and species.
Fig. 3: S. alvi builds biomass from substrates derived from the glucose metabolism of the host.  a, Experimental design of the pulse–chase isotope labelling gnotobiotic bee experiment. Newly emerged MF bees were fed 100% 13C glucose for 4 days (96 h), colonized with a defined quantity of S. alvi and then the 13C glucose was replaced by 12C glucose 24 h after colonization. The timeline indicates when bees were sampled for c.f.u. plating, GC–MS and NanoSIMS analysis relative to the timepoint of colonization (0 h). All bees were from the same hive. b, Colonization levels of S. alvi in the ileum until 72 h postinoculation show the initial strong colonization bottleneck and subsequent exponential increase in cell numbers. Colonization success is indicated as percentage of bees with detectable c.f.u. Pink squares indicate the inoculum of S. alvi (OD = 0.1). The LOD of 70 c.f.u. per ileum is indicated with the dashed line. c, The average at.% 13C enrichments of S. alvi cells decreases rapidly, while the enrichment of host cells and epithelium remains constant over 72 h in ileum cross-sections imaged with NanoSIMS. Each data point represents the mean of ROI; bacterial ROIs consist of single bacterial cells, while epithelium and host cell ROIs represent the total area encompassing epithelium or host cell tissue in each image. ROI raw counts and calculated enrichments are listed in Supplementary Table 3. The dashed line at 1.1% indicates the natural 13C abundance calculated from NanoSIMS images of 12C control bees. d, 13C enrichment of metabolites in the gut steadily decreases in the 12C chase phase. Dots represent the average 13C enrichment of a given metabolite across the bees sampled at that timepoint. Average values and colours are shown by metabolite class: carboxylic acids (blue), non-essential amino acids (NEAA, green), essential amino acids (EAA, yellow). Shaded regions encompass the maximum and minimum limits of each metabolite class, n = 3 bees per timepoint. Measured metabolites are listed in Supplementary Table 3. The dotted line at 1.1% indicates the natural 13C abundance. e, Schematic drawing of the honey bee gut shows the region in the ileum (black bar) where cross-sections were taken, partially adapted from ref. 12. TEM images and corresponding NanoSIMS images of two different 13C-labelled bees at 48 and 72 h after inoculation. White arrows indicate S. alvi cells. The at.% 13C represents percentage of 13C atoms; the natural 13C abundance is ~1.1 at.%. Scale bars, 5 μm.
a, Experimental design of the pulse–chase isotope labelling gnotobiotic bee experiment. Newly emerged MF bees were fed 100% 13C glucose for 4 days (96 h), colonized with a defined quantity of S. alvi and then the 13C glucose was replaced by 12C glucose 24 h after colonization. The timeline indicates when bees were sampled for c.f.u. plating, GC–MS and NanoSIMS analysis relative to the timepoint of colonization (0 h). All bees were from the same hive. b, Colonization levels of S. alvi in the ileum until 72 h postinoculation show the initial strong colonization bottleneck and subsequent exponential increase in cell numbers. Colonization success is indicated as percentage of bees with detectable c.f.u. Pink squares indicate the inoculum of S. alvi (OD = 0.1). The LOD of 70 c.f.u. per ileum is indicated with the dashed line. c, The average at.% 13C enrichments of S. alvi cells decreases rapidly, while the enrichment of host cells and epithelium remains constant over 72 h in ileum cross-sections imaged with NanoSIMS. Each data point represents the mean of ROI; bacterial ROIs consist of single bacterial cells, while epithelium and host cell ROIs represent the total area encompassing epithelium or host cell tissue in each image. ROI raw counts and calculated enrichments are listed in Supplementary Table 3. The dashed line at 1.1% indicates the natural 13C abundance calculated from NanoSIMS images of 12C control bees. d, 13C enrichment of metabolites in the gut steadily decreases in the 12C chase phase. Dots represent the average 13C enrichment of a given metabolite across the bees sampled at that timepoint. Average values and colours are shown by metabolite class: carboxylic acids (blue), non-essential amino acids (NEAA, green), essential amino acids (EAA, yellow). Shaded regions encompass the maximum and minimum limits of each metabolite class, n = 3 bees per timepoint. Measured metabolites are listed in Supplementary Table 3. The dotted line at 1.1% indicates the natural 13C abundance. e, Schematic drawing of the honey bee gut shows the region in the ileum (black bar) where cross-sections were taken, partially adapted from ref. 12. TEM images and corresponding NanoSIMS images of two different 13C-labelled bees at 48 and 72 h after inoculation. White arrows indicate S. alvi cells. The at.% 13C represents percentage of 13C atoms; the natural 13C abundance is ~1.1 at.%. Scale bars, 5 μm.
Quinn, A., El Chazli, Y., Escrig, S. et al.
Host-derived organic acids enable gut colonization of the honey bee symbiont Snodgrassella alvi.
Nat Microbiol (2024). https://doi.org/10.1038/s41564-023-01572-y
Copyright: © 2024 The authors.
Published by Springer Nature Ltd. Open access.
Reprinted under a Creative Commons Attribution 4.0 International license (CC BY 4.0)
ID is not a problem for science; rather science is a problem for ID. This book shows why. It exposes the fallacy of Intelligent Design by showing that, when examined in detail, biological systems are anything but intelligently designed. They show no signs of a plan and are quite ludicrously complex for whatever can be described as a purpose. The Intelligent Design movement relies on almost total ignorance of biological science and seemingly limitless credulity in its target marks. Its only real appeal appears to be to those who find science too difficult or too much trouble to learn yet want their opinions to be regarded as at least as important as those of scientists and experts in their fields.
Available in Hardcover, Paperback or ebook for Kindle
This book explains why faith is a fallacy and serves no useful purpose other than providing an excuse for pretending to know things that are unknown. It also explains how losing faith liberates former sufferers from fear, delusion and the control of others, freeing them to see the world in a different light, to recognise the injustices that religions cause and to accept people for who they are, not which group they happened to be born in. A society based on atheist, Humanist principles would be a less divided, more inclusive, more peaceful society and one more appreciative of the one opportunity that life gives us to enjoy and wonder at the world we live in.
Available in Hardcover, Paperback or ebook for Kindle





No comments :
Post a Comment
Obscene, threatening or obnoxious messages, preaching, abuse and spam will be removed, as will anything by known Internet trolls and stalkers, by known sock-puppet accounts and anything not connected with the post,
A claim made without evidence can be dismissed without evidence. Remember: your opinion is not an established fact unless corroborated.