
Most biologists now accept the Endosymbiosis Theory which explains how simple prokaryote cells became complex eukaryote cells by a single-celled prokaryote such as an archaea incorporating other single-celled prokaryotes inside its cell membrane. This may have been by engulfing them as prey or by being parasitised by them. Whatever the mechanism, a symbiotic relationship ensued which progressed to the extent that the incorporated cell's DNA was transferred to the host genome and the incorporated cell became a cell organelle.
This explains the origin of cell organelles such as the mitochondria which metabolise glucose to turn adenosine diphosphate (ADP) into adenosine triphosphate (ATP) which can then be used to power metabolic processes within the cell. Mitochondria have some similarities with rickettsia bacteria which strongly suggests they have evolved from free-living rickettsia.
Likewise, chloroplasts in plant cells were once free-living, photosynthesising cyanobacteria which became incorporated in what was to become algae, so giving rise eventually to almost all plant life.
And now we have evidence that another incorporation is evolving, in the form of nitrogen-fixing bacteria being incorporated as organelles into a marine alga, which gives the algae the ability to create ammonia and so nitrates directly from atmospheric nitrogen. This was discovered by researchers from the University of Rhode Island, the Institut de Ciències del Mar in Barcelona, the University of California at Santa Cruz and the Massachusetts Institute of Technology. They have published their findings, open access, in the journal Cell.
Although nitrogen is abundant, comprising about 79% of Earth's atmosphere, it exists as the diatomic gas dinitrogen (N2) which is notoriously stable making molecular nitrogen almost an inert substance and requiring a lot of energy to break the N-N bond. However, some bacteria, the nitrogen-fixing bacteria, have evolved the ability to do this using the enzyme nitrogenase:
What is the biochemical pathway which converts molecular nitrogen into ammonia? The biochemical pathway that converts molecular nitrogen (N2) into ammonia (NH3) is called nitrogen fixation. Nitrogen fixation is a complex process that is primarily carried out by certain bacteria, such as Rhizobia in the root nodules of leguminous plants or free-living nitrogen-fixing bacteria like Azotobacter and cyanobacteria.Some of the bacteria which perform this function are known to science as Unicellular Cyanobacterium Group A (UCYN-A), members of the Chroococcales order of cyanobacteria. These unicellular cyanobacteria are unique in their ability to form symbiotic relationships with certain marine phytoplankton species, particularly various types of diatoms. They form symbiotic associations with their host phytoplankton, where they provide fixed nitrogen (ammonium) in exchange for photosynthetically fixed carbon compounds from the host.
The key enzyme responsible for catalyzing the conversion of molecular nitrogen into ammonia is nitrogenase. Nitrogenase is a complex enzyme system composed of multiple proteins, including nitrogenase reductase and nitrogenase iron protein, which are involved in the reduction of nitrogen.
The overall reaction catalyzed by nitrogenase can be represented as follows:
N2 + 8H+ + 8e- + 16ATP → 2NH3 + H2 + 16ADP + 16Pi (phosphate).
In this reaction, molecular nitrogen (N2) is reduced to ammonia (NH3) through the addition of electrons (e-) and protons (H+) in the presence of ATP (adenosine triphosphate) as an energy source. The process also generates hydrogen gas (H2).
The reduction of nitrogen by nitrogenase is a highly energy-intensive process, requiring the input of ATP to drive the reaction. Additionally, nitrogenase is highly sensitive to oxygen, so nitrogen-fixing bacteria have evolved mechanisms to protect the enzyme from oxygen, such as residing in anaerobic environments or producing specialized structures like root nodules.
Overall, nitrogen fixation plays a crucial role in the global nitrogen cycle by converting atmospheric nitrogen into a form (ammonia) that can be utilized by plants and other organisms, ultimately contributing to the fertility of ecosystems.
It is these bacteria that the researchers believe may be in the process of evolving into cell organelles within the algae, Braarudosphaera bigelowii. Their research is explained in a University of Rhode Island news item:
Nitrogen is a nutrient essential for all life on Earth. Although nitrogen gas (N2) is plentiful, it is largely unavailable to most organisms without a process known as nitrogen fixation, which converts dinitrogen to ammonium—a major inorganic nitrogen source.Technical details appear in the team's open access paper in Cell:
While there are bacteria that are able to reduce dinitrogen to ammonium, researchers at the University of Rhode Island, Institut de Ciències del Mar in Barcelona, University of California at Santa Cruz and the Massachusetts Institute of Technology have discovered nitrogen-fixing symbiotic organisms exhibiting behaviors similar to organelles. In fact, researchers posit these symbiotic organisms – UCYN-A, a species of cyanobacteria – may be evolving organelle-like characteristics. Their study was recently published in the journal Cell.
UCYN-A live in a symbiotic relationship with a closely related group of marine algae, B. bigelowii, in areas of the open ocean that are often low in nutrients. Most nitrogen-fixing bacteria have mechanisms to regulate dinitrogen use when fixed sources of nitrogen are available, alleviating the high energetic cost of this process. However, UCYN-A have lost the genes allowing this and are able to fix nitrogen gas into ammonium even in nutrient-rich environments. The host, in-turn, provides it with carbon fixed photosynthetically by its chloroplasts.
The study details how researchers found a size relationship between UCYN-A and their symbiotic partner cells – consistent with the size relationships between other organelles and their hosts. As organelles get larger, so do their host cells – eventually dividing and replicating. Mathematical modeling revealed the metabolic trade-offs which regulate the relative cell size through nutrient acquisition and exchange.
While organelles such as mitochondria and chloroplasts are much further along on the evolutionary spectrum, researchers contend that what they are seeing may be a snapshot of the evolutionary process of bacterial-derived organelles that are nitrogen-fixing.It requires lots of energy as well as electrons to fix nitrogen gas, to make it into something useful. If UCYN-A are moving along the evolutionary path toward developing into nitrogen-fixing organelles and we find cells aside from B. bigelowii also have such organelles, or are evolving similarly, it could be a game-changer.
Assistant Professor Keisuke Inomura, co-lead author
Graduate School of Oceanography
University of Rhode Island, Narragansett, RI, USA.Researchers note, however, that more study is needed to demonstrate whether this is the case.Our study focuses on a much more recent symbiotic relationship that emerged about 100 million years ago, allowing us to explore the evolution of organelle formation in its early stages.
Dr Francisco Cornejo, co-lead author
Department of Marine Biology and Oceanography
Institut de Ciències del Mar, ICM-CSIC, Barcelona, Spain.The surprisingly tight size relationship between UCYN-A and its host can be explained by the resource economy of the partners. It suggests that UCYN-A may be on the path to becoming an organelle: whether it may already be so is the subject of ongoing research.
Professor Michael J. Follows, senior author
Department of Earth, Atmospheric, and Planetary Sciences
Massachusetts Institute of Technology, Cambridge, MA, USA.
HighlightsI wonder how creationist dogma, which insists there are two different sorts of evolution, 'microevolution' and 'macroevolution' - the latter being deemed 'impossible', would fit this evolution of a new cell organelle into that classification. Biology has no such difficulty, of course because it recognises that the cell organelles are the result of the evolution of former free-living single-celled prokaryotes and that eukaryotes are themselves the result of the evolution of prokaryotes by forming symbiotic alliances. There is no need to force-fit those forms of evolution into the straight-jacket creationism tries to impose on science to make it easier to attack.
- Strong volume covariation in the B. bigelowii/UCYN-A nitrogen-fixing symbiosis
- The constant volumetric relationship reflects symbiotic resource economy constraints
- Coordination of harvesting and exchange of resources maximizes symbiotic growth rate
- UCYN-A is functioning like a hypothetical N2-fixing organelle (or nitroplast)
Summary
Biological dinitrogen (N2) fixation is a key metabolic process exclusively performed by prokaryotes, some of which are symbiotic with eukaryotes. Species of the marine haptophyte algae Braarudosphaera bigelowii harbor the N2-fixing endosymbiotic cyanobacteria UCYN-A, which might be evolving organelle-like characteristics. We found that the size ratio between UCYN-A and their hosts is strikingly conserved across sublineages/species, which is consistent with the size relationships of organelles in this symbiosis and other species. Metabolic modeling showed that this size relationship maximizes the coordinated growth rate based on trade-offs between resource acquisition and exchange. Our findings show that the size relationships of N2-fixing endosymbionts and organelles in unicellular eukaryotes are constrained by predictable metabolic underpinnings and that UCYN-A is, in many regards, functioning like a hypothetical N2-fixing organelle (or nitroplast).
Introduction
Nitrogen (N) is essential for life on Earth. Despite the large reservoir of atmospheric dinitrogen (N2) gas, primary producers depend on “fixed” forms of N such as nitrate or ammonium, which can be largely limiting in ecosystems such as the oligotrophic open ocean. Only some Bacteria and Archaea (N2-fixers or diazotrophs) are able to reduce N2 to ammonium (N2 fixation), which makes them essential for the maintenance of primary productivity in diverse ecosystems.1,2,3 However, N2 fixation is also widespread in eukaryotes through symbioses with diazotrophs, including some cyanobacteria, which are common in aquatic and terrestrial environments.1,4
One symbiotic N2-fixing bacterium is the cyanobacterium Candidatus Atelocyanobacterium thalassa (hereafter, UCYN-A), which might be evolving organelle-like characteristics. Natural populations of closely related sublineages of UCYN-A live as endosymbionts with a closely related group of species of the haptophyte microalga Braarudosphaera bigelowii,2,5,6 all uncultivated except for one set of coastal strains6 (Figure 1A). UCYN-A are phylogenetically related to cyanobacteria but have a greatly reduced genome and are metabolically streamlined, having lost the enzymes needed for carbon (C) fixation, the tricarboxylic acid cycle, and photosystem II that is necessary for oxygenic photosynthesis in cyanobacteria.7 On the other hand, UCYN-A have retained the entire set of N2 fixation genes and provide fixed N to their hosts, which in turn provide UCYN-A with C fixed photosynthetically by its chloroplasts.2 B. bigelowii and its closely related species are eukaryotic calcifying algae with two chloroplasts per cell8 that have both calcareous and naked flagellate life stages, and one UCYN-A spheroid body is physically located within the host cell between the two chloroplasts in the B. bigelowii calcified form5 and at the posterior side of the naked flagellate form in culture6 (Figure 1A). UCYN-A is surrounded by a single host membrane, engulfed in what is thought to be the host’s food vacuole.5,6,9 In the open ocean and coastal environments, closely related species of B. bigelowii display a large variation in cell length (10-fold, from 2 to 3 μm up to 20–30 μm) and harbor genetically distinct but closely related UCYN-A sublineages (UCYN-A1, -A2, and -A3 sublineages) (Figure 1C), which also range 10-fold in effective cell radius and up to ca. 1,000-fold in cell volume.5,10,11,12 There is significant empirical evidence that the per-cell photosynthesis rate of diverse, marine free-living phytoplankton taxa, including haptophytes, exhibits power-law relationships with cell volume.13,14,15,16,17 However, it is unclear whether this allometric relationship holds in phytoplankton taxa harboring endosymbionts that fully depend on the C fixation of their hosts, which is the case with UCYN-A.18 On the other hand, it is also unknown whether N2 fixation in UCYN-A affects the cell size of their haptophyte symbiotic partners in an analogous way to photosynthesis in marine phytoplankton. The large cell size variation that is observed across the multiple closely related UCYN-A lineages and B. bigelowii species in symbiotic partnerships provides a unique opportunity to explore the metabolic underpinnings of these host/symbiont cell size relationships and, ultimately, might explain the role of size in the evolution of symbiotic interactions.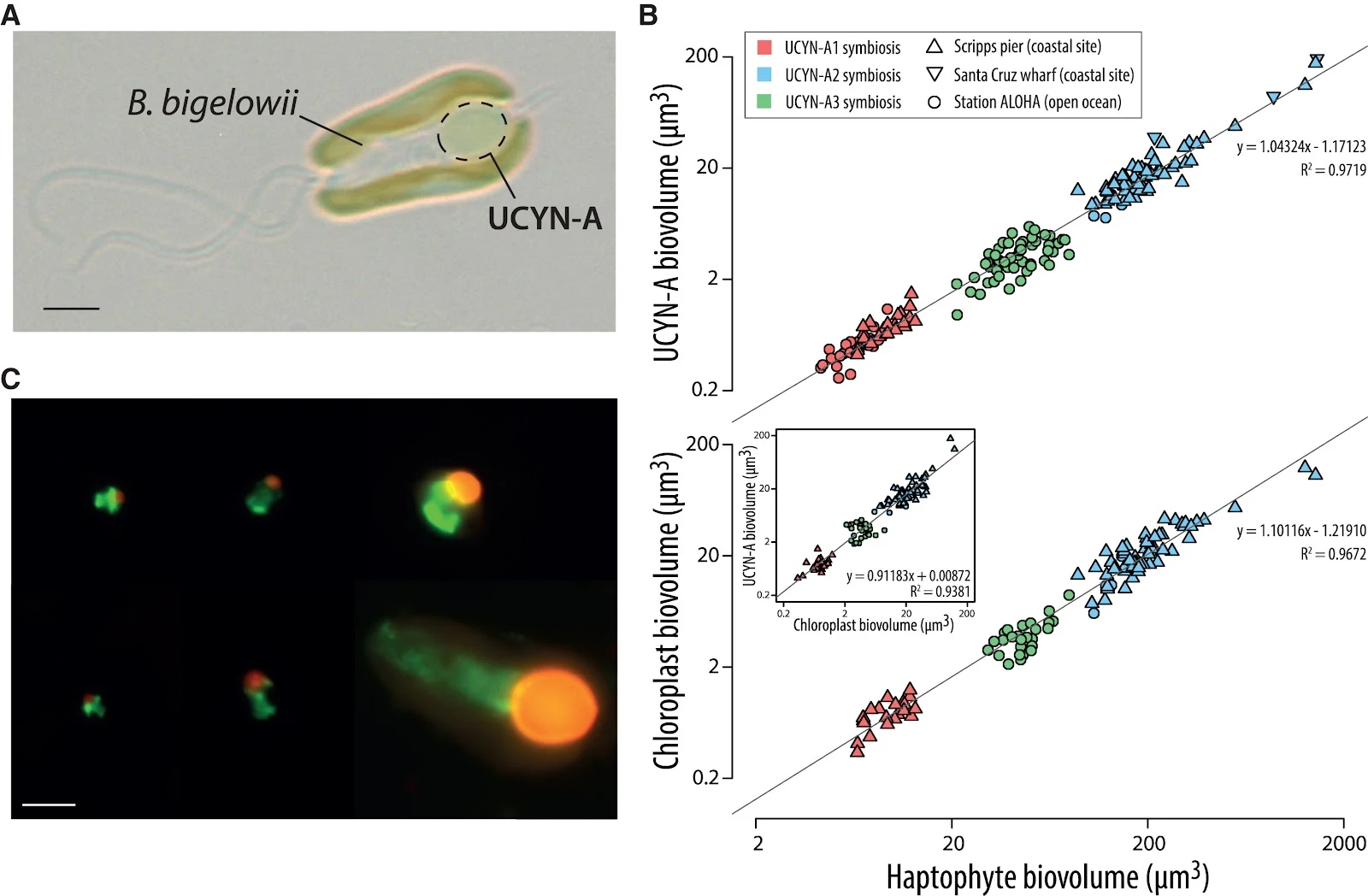 Figure 1 Linear volumetric scaling in the B. bigelowii/UCYN-A symbiosis
Figure 1 Linear volumetric scaling in the B. bigelowii/UCYN-A symbiosis
(A) Light microscopy image of the haptophyte B. bigelowii in symbiosis with UCYN-A (scale bar, 2 μm; image courtesy of Wing Kwan Esther Mak and Kyoko Hagino).
(B) Upper panel: UCYN-A volume (sublineages UCYN-A1, -A2, and -A3; see inset) as a function of associated haptophyte cell volume; lower panel: chloroplast volume as a function of associated haptophyte cell volume; inset in lower panel: UCYN-A volume vs. chloroplast volume of associated host cells.
(C) Composite of catalized reporter deposition fluorescence in situ hybridization (CARD-FISH) images showing the range of sizes of the different UCYN-A sublineages (left images: UCYN-A1 sublineage; middle images: UCYN-3 sublineage; right images: UCYN-A2 sublineage) and their symbiotic haptophyte partners. Images were taken under blue (green-labeled haptophytes with Alexa 488 stain) and green (red-labeled UCYN-A with Alexa 594 stain) excitation wavelengths (STAR Methods) (scale bar, 5 μm).
See also Figure S1.
 Figure 2 The metabolic model predicts the optimal size ratio for the B. bigelowii/UCYN-A symbiosis
Figure 2 The metabolic model predicts the optimal size ratio for the B. bigelowii/UCYN-A symbiosis
(A) Conceptual view of resource acquisition and exchange in the B. bigelowii/UCYN-A symbiosis.
(B) Predicted growth rate of the symbiosis, μ (d−1), vs. ratio of the effective spherical radii of the haptophyte and UCYN-A, RH/RD. The solid blue line indicates the predicted growth rate for the symbiotic pair when nothing limits the growth but the C provided through the haptophyte photosynthesis across different host-symbiont size ratios, and the blue shading indicates the range inferred by 1 standard deviation in the C-specific photosynthesis rate of haptophytes in this size range.21 The red line is the modeled maximum growth rate when nothing limits the growth but the N provided by UCYN-A through N2 fixation, calibrated by the maximum observed N-specific N2 fixation rate in UCYN-A.18 The lowest of these two growth rates is the achievable growth rate (black dash dotted line). The highest synchronized growth rate occurs at the radius ratio where the blue and red lines cross (RH/RD)opt (black circle).
(C) Distribution of the observed ratio of the radii of haptophyte cells and UCYN-A for each individual symbiotic pair within and across UCYN-A sublineages. N is the number of measured symbiotic pairs. The dashed vertical line and gray shading indicate the predicted optimal ratio from the model depicted in (B) and its range relative to one standard deviation in the empirical maximum photosynthesis rate.
See also Figure S2.
Cornejo-Castillo, Francisco M.; ; Inomura, Keisuke; Zehr, Jonathan P.; Follows, Michael J.
Metabolic trade-offs constrain the cell size ratio in a nitrogen-fixing symbiosis
Cell (2024) 187(7) 1762-1768.e9; DOI: 10.1016/j.cell.2024.02.016.
Copyright: © 2024 The authors.
Published by Elsevier Inc. Open access.
Reprinted under a Creative Commons Attribution 4.0 International license (CC BY 4.0)
Of course, anything happening at the unicellular level is flummoxing for creationism because the ignorant hill farmers who made up their sacred source of science knew nothing about microorganisms or anything smaller than their naked, unaided eyes could see, so they saw no need to include prokaryote and eukaryote cells in any of the tales they made up to fill the void in their knowledge and understanding of the world around them, just as they saw no need to explain atoms, galaxies and elementary particles or include them in their tales.
What Makes You So Special? From The Big Bang To You
Ten Reasons To Lose Faith: And Why You Are Better Off Without It






No comments :
Post a Comment
Obscene, threatening or obnoxious messages, preaching, abuse and spam will be removed, as will anything by known Internet trolls and stalkers, by known sock-puppet accounts and anything not connected with the post,
A claim made without evidence can be dismissed without evidence. Remember: your opinion is not an established fact unless corroborated.