SARS-CoV-2 “steals” our proteins to protect itself from the immune system
Although COVD-19 has been mostly brought under control by medical science and the vaccination campaign, it still kills thousands of people a year, but nowhere near the volume of deaths during the initial wave when world-wide health services came close to collapse and economies were on the point of ruin.
But there is still much to learn about why it was so virulent and successful.
To an admirer of creationism’s divine malevolence it must have seemed like a triumph of design, as it filled hospitals, killed millions and wrecked economies, helped by its supporters in the evangelical Christian churches who opposed measures to mitigate the worse effect of the virus, and then opposed the vaccination campaign with lies, scare tactics and the most infantile conspiracy theories imaginable, to help ensure the virus got to as many people as possible.
Now, a team of researcher from the Medical University of Vienna together with colleagues from the Medical University of Innsbruck have discovered how the virus protects itself from the immune system creationists believe their putative intelligent designer designed to protect us from the virus’s and other pathogens it designs to make us sick, would grace the pages of another 'intelligent design' polemic by Michael J. Behe and his Deception Institute. It depends on several components of a system being present in a classic 'irreducibly complex' system that creationists wave around as 'proof' that the locally-popular creator god is real because they can't understand how it could have evolved.
Tell me about the complement system of immune response to viral infections. The complement system is a crucial part of the immune response, providing a rapid defense against viral infections. It consists of a series of proteins in the blood and tissues that work in a cascade to detect, mark, and eliminate pathogens, including viruses. The complement system enhances the ability of antibodies and immune cells to clear infections, thus “complementing” the adaptive immune response.This argument from ignorant incredulity and false dichotomy fallacy is as good as it gets from creationists and is the whole basis of several books by Behe.
Key Roles of the Complement System in Viral DefenseComplement Pathways in Viral Infection
- Direct Viral Neutralization: Some complement proteins can bind directly to the surface of viral particles, blocking their ability to attach to and enter host cells. This effectively neutralizes the virus before it can cause an infection.
- Opsonization and Phagocytosis: The complement system marks pathogens for phagocytosis through a process called opsonization. Proteins like C3b coat the virus, making it easier for phagocytic cells, such as macrophages and neutrophils, to recognize and engulf the pathogen.
- Formation of the Membrane Attack Complex (MAC): Through a sequence of activations, complement proteins can form a pore-like structure called the MAC, which directly inserts into the membrane of some enveloped viruses or infected cells. This leads to lysis, essentially puncturing the viral or cell membrane and killing the pathogen.
- Enhancing Inflammatory Responses: Certain complement fragments (e.g., C3a, C5a) act as anaphylatoxins, which can attract immune cells to the site of infection and promote inflammation. This response can help localize the infection and increase the immune system’s ability to clear the virus.
- Bridging Innate and Adaptive Immunity: The complement system aids in bridging the innate and adaptive immune responses. For example, by enhancing antigen presentation, the complement system helps stimulate a stronger, more effective adaptive immune response, particularly by supporting the activation of B cells, which produce antibodies against the virus.
The complement system operates through three main activation pathways:
- Classical Pathway: Triggered by antibodies bound to viral antigens. This is often engaged after the adaptive immune system has generated specific antibodies against the virus.
- Alternative Pathway: Activated directly by viral surface proteins or other pathogen-associated molecular patterns (PAMPs) without the need for antibodies. This pathway can provide an immediate defense during early infection stages.
- Lectin Pathway: Initiated by binding of mannose-binding lectin (MBL) to carbohydrates on viral surfaces. This pathway also functions independently of antibodies and is part of the early innate response.
Viral Evasion of the Complement System
Viruses have evolved strategies to evade the complement system. Some produce proteins that mimic host regulatory proteins, which inhibit complement activation on their surfaces. Others incorporate host cell membrane proteins into their viral envelope to escape recognition.
In sum, the complement system is an essential first-line defense against viruses. Its actions help control viral spread, support inflammation, and prepare the adaptive immune system to mount a targeted response. However, viral evasion mechanisms highlight the dynamic interplay between host defenses and viral adaptation.
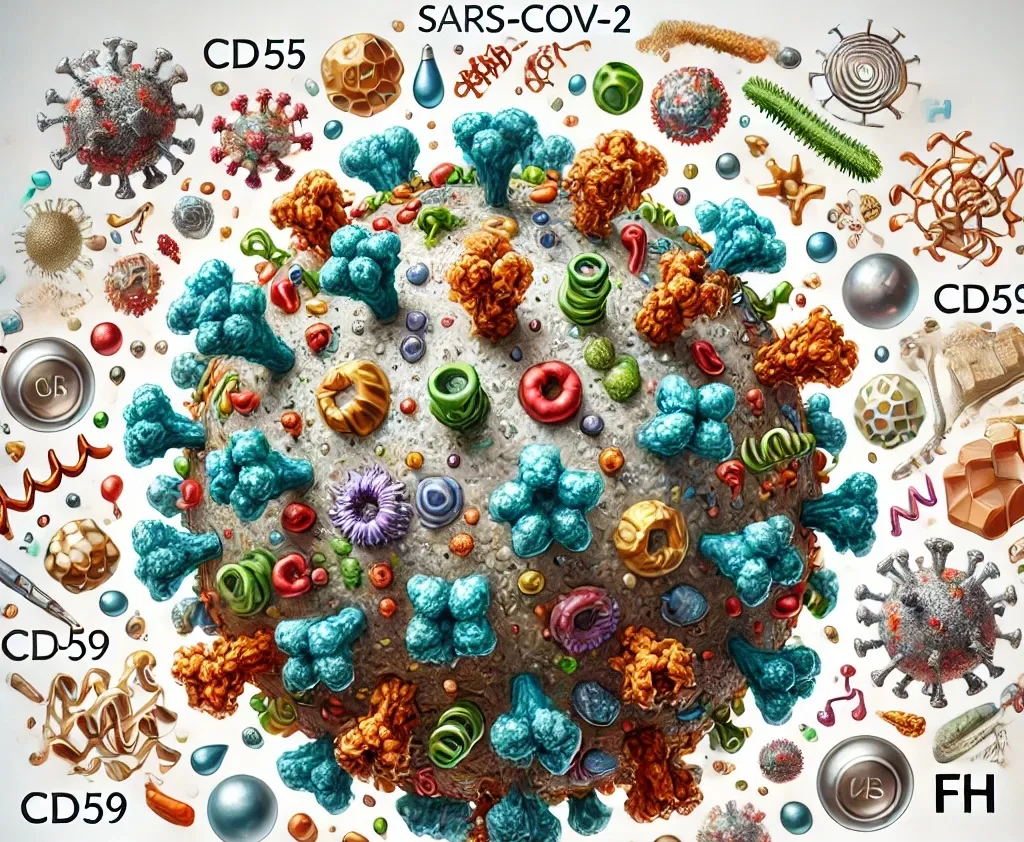
The Austrian researchers have discovered that the SARS-CoV-2 virus that causes COVID-19 steals three proteins that are part of the complement regulatory system. The compliment system is the initial response to virus infection that binds to the virus particles and prepares them for destruction. The regulatory system deactivates the compliment system to prevent it damaging host tissues.
By coating itself in these proteins, the SARS-CoV-2 virus effectively neutralises the complement system so abolishing the first line of defence. The team of researchers have recently published their finding, open access, in the journal Emerging Microbes & Infections, and explained their research in a
Medical University of Vienna news item:
SARS-CoV-2 “steals” our proteins to protect itself from the immune system
Virus hijacks three important host proteins that dampen the activity of the complement system
(Vienna/Innsbruck, 08 November 2024) Researchers at the Medical University of Vienna and the Medical University of Innsbruck discovered that SARS-CoV-2 hijacks three important host proteins that dampen the activity of the complement system, a key component of early antiviral immunity. This significantly impairs viral clearance which may affect the course of both acute COVID-19 infections and post-COVID-19 sequelae. The study was recently published in the journal “Emerging Microbes & Infections”.
An early and effective immune response is crucial for resolving viral infections and preventing post-infectious complications. The complement system, a pivotal element of antiviral immunity, is a cascade of proteins found in the bloodstream and at mucosal sites, such as the respiratory tract. Activated through three different pathways, complement facilitates the clearance of virus particles by directly inducing their destruction (lysis). To prevent bystander damage to host cells, complement is rapidly inactivated by a set of host molecules referred to as complement regulatory proteins. The new study led by Anna Ohradanova-Repic and colleagues from the Center for Pathophysiology, Infectiology and Immunology at the Medical University of Vienna in collaboration with the team of Heribert Stoiber from the Institute of Virology at the Medical University of Innsbruck shows that SARS-CoV-2 hijacks three of these regulatory proteins, CD55, CD59 and Factor H, and thereby successfully shields itself from complement-mediated lysis.
Hijacking host proteins for effective complement resistance
By propagating SARS-CoV-2 in human cells the researchers discovered that the virus particles acquire the cellular proteins CD55 and CD59. Further experiments showed that SARS-CoV-2 also binds to Factor H, another complement regulatory protein that is primarily found in the bloodstream. Confronting the virus particles with active complement revealed that they are partially resistant to complement-mediated lysis. By removing CD55, CD59 and Factor H from the virus surface or inhibiting their biological functions, the researchers could successfully restore complement-mediated clearance of SARS-CoV-2.
Through hijacking these three proteins, SARS-CoV-2 can evade all three complement pathways, resulting in reduced or delayed viral clearance by the infected host.
Anna Ohradanova-Repic, co-corresponding author
Medical University of Vienna
Center for Pathophysiology, Infectiology and Immunology,
Institute for Hygiene and Applied Immunology, Vienna, Austria.
Because complement is intricately linked with other components of the immune system, this not only affects virus elimination but can also cause significant inflammation, a core feature of both severe COVID-19 and Long COVID.
Uncovering immune evasion mechanisms that allow the virus to linger within the host for longer, deepen our understanding of the acute and long-term impacts of SARS-CoV-2 infection.
Laura Gebetsberger, first author
Medical University of Vienna
Center for Pathophysiology, Infectiology and Immunology,
Institute for Hygiene and Applied Immunology, Vienna, Austria.
Publication:
ABSTRACTGiven the SARS-CoV-2 virus's ability to mutate to stay ahead of the game in its arms race between it and our immune system aided by medical science, none of which can be sensibly portrayed as 'devolution' from some hypothetical initial intelligently created perfection, and given that it is irreducibly complex in that it can’t work as a virus if important parts are missing, advocates of intelligent design or 'cdesign proponencists' are left with explaining a couple of embarrassing things:
The complement system is a vital anti-microbial defence mechanism against circulating pathogens. Excessive complement activation can have deleterious outcomes for the host and is consequently tightly modulated by a set of membrane-associated and fluid-phase regulators of complement activation (RCAs). Here, we demonstrate that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) hijacks host cellular RCA members CD55 and CD59 and serum-derived Factor H (FH) to resist antibody-dependent complement-mediated lysis triggered by immunized human sera. Blockage of the biological functions of virion-associated CD55 and CD59 and competition of FH recruitment with functionally inactive recombinant FH-derived short consensus repeats SCR18-20 restore SARS-CoV-2 complement sensitivity in a synergistic manner. Moreover, complement-mediated virolysis is dependent on classical pathway activation and does not occur in the absence of virus-specific antibodies. Altogether, our findings present an intriguing immune escape mechanism that provides novel insights into the immunopathology observed in severe coronavirus disease 2019 (COVID-19).
Introduction
The complement system is an integral first-line defence mechanism against invading pathogens and comprises > 30 proteins in plasma, on cell surfaces or within host cells [1]. Complement operates in three pathways with distinct patterns of activation, the classical (CP), lectin (LP) and alternative pathways (AP), and results in three major outcomes: (i) opsonization and phagocytosis, (ii) chemotaxis of inflammatory immune cells and (iii) direct pathogen lysis [1,2]. The CP is initiated when the complement component C1q interacts with IgM or IgG immune complexes, or pathogen-associated molecular patterns on pathogen surfaces, leading to the activation of the multimeric C1 complex (C1qCr2Cs2) [2]. Recognition of carbohydrate motifs on non-self surfaces by mannose binding lectin (MBL), collectins and ficolins activates the LP, and the AP is fuelled by the constant low-grade hydrolysis (“tick-over”) of C3 to C3(H2O), or by C3b via the amplification loop (AL) [1–3]. All three pathways converge on the formation of a C3 convertase, which cleaves the central complement component C3. This generates C3b, which promotes pathogen opsonization and phagocytosis and further drives the formation of the C5 convertases, which cleave C5 and thereby activate the terminal pathway. In this, the generated C5b sequentially associates with C6, C7, C8 and multiple C9 molecules and assembles the membrane attack complex (MAC, C5b-9), a small pore that triggers pathogen lysis by disrupting the integrity of cell membranes or viral envelopes [1,2]. The cleavage of C3 and C5 further produces the anaphylatoxins C3a and C5a, which initiate potent inflammatory responses through the chemotaxis of immune cells via their cognate receptors C3aR, C5aR and C5L2 [2].
To prevent bystander damage to host cells, complement is tightly regulated by both fluid-phase and membrane-associated proteins (“regulators of complement activation,” RCAs) [3]. These include the plasma membrane-resident protectin (CD59) and decay accelerating factor (DAF, CD55) as well as the fluid-phase regulator Factor H (FH), which shield self surfaces from complement-mediated injury through (i) inhibiting MAC formation (CD59), (ii) destabilizing CP/LP and/or AP C3 convertases (“decay acceleration,” CD55, FH), and (iii) exerting cofactor activity for Factor I-mediated C3b inactivation (FH) [3,4]. Importantly, numerous pathogens have subverted these regulatory mechanisms through various strategies, including (i) the hijacking of host RCAs, (ii) enzymatic inactivation of host complement factors, and (iii) the production of complement-like proteins which mimic RCA functions [5,6].
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the aetiological agent of coronavirus disease 2019 (COVID-19), is a large enveloped positive-sense single-stranded RNA virus in the Coronaviridae family [7]. The viral genome encodes four structural proteins, the core nucleocapsid (N) and surface spike (S), envelope (E) and membrane (M) proteins, as well as 16 non-structural (nsp) and 9 accessory ORF proteins, which assist viral replication [7,8]. Clinical manifestations of SARS-CoV-2 infection are highly variable, ranging from mild upper respiratory tract symptoms in most patients to severe disease characterized by an uncontrolled state of hyperinflammation, acute respiratory distress syndrome (ARDS), multiorgan failure and death [9,10]. Complement activation has been heavily implicated in the pathogenesis of severe COVID-19, reflected by elevated levels of C3a, C5a, and soluble C5b-9 (sC5b-9) in the plasma of critically ill patients, as well as the deposition of activated complement products in injured organs [10–14]. The crosstalk between complement and coagulation has been further associated with the COVID-19-mediated coagulopathy and thromboinflammation [11,15,16].
Despite the extensive research on the role of complement in COVID-19, not much is known about the interaction of SARS-CoV-2 with RCAs. Here, we demonstrate that SARS-CoV-2 hijacks host cellular CD55 and CD59 as well as serum-derived FH to resist antibody-dependent complement-mediated lysis triggered by immunized human sera. Blockage of the biological functions of virion-incorporated CD55 and CD59 and inhibition of FH recruitment restore the complement sensitivity of SARS-CoV-2 in a synergistic manner. Moreover, complement-mediated virolysis seems to depend on CP activation and is not observed in the absence of virus-specific antibodies. Altogether, we present an intriguing immune escape mechanism, which may contribute to the complement-driven pathology observed in severe COVID-19.
Firstly, why, if irreducible complexity is 'proof' of intelligent design why the irreducible complexity of the SARS-CoV-2 virus shouldn't also be regarded as 'proof' that it was designed by their putative intelligent designer.
Secondly, why would any intelligent designer design an immune system to protect us from the viruses it created to make us sick, then design the viruses to usurp part of that immune system to protect itself and evade an immune response?
And lastly, a question I have asked repeatedly and never had an answer to, why is it preferable for people to believe the god of the Bible and Qur'an is a pestilential malevolence who creates ways to increase the suffering in the world, than to accept that the Theory of Evolution is a better explanation for parasites and pathogens which absolves gods of any responsibility for the misery they cause?
Refuting Creationism: Why Creationism Fails In Both Its Science And Its Theology
A systematic refutation of creationism, exposing the childish parodies, fallacies and pseudo-science that underpins it. This book takes the reader through the major themes of creationism from the Big Bang, through abiogenesis and the origin of the genetic code, the evidence for evolution, falsifying the claims of creationism with scientific evidence. Also exposed is the deliberate disinformation to be found on creationist websites and publications and the absurdity of the belief that the Bible, particularly the origin myths in Genesis, is real science and genuine history, written or inspired by an omniscient, inerrant creator god
Available in Hardcover, Paperback or ebook for Kindle
This book presents the reader with multiple examples of why, even if we accept Creationism's putative intelligent designer, any such entity can only be regarded as malevolent, designing ever-more ingenious ways to make life difficult for living things, including humans, for no other reason than the sheer pleasure of doing so. This putative creator has also given other creatures much better things like immune systems, eyesight and ability to regenerate limbs that it could have given to all its creation, including humans, but chose not to. This book will leave creationists with the dilemma of explaining why evolution by natural selection is the only plausible explanation for so many nasty little parasites that doesn't leave their creator looking like an ingenious, sadistic, misanthropic, malevolence finding ever more ways to increase pain and suffering in the world, and not the omnibenevolent, maximally good god that Creationists of all Abrahamic religions believe created everything. As with a previous book by this author, "The Unintelligent Designer: Refuting the Intelligent Design Hoax", this book comprehensively refutes any notion of intelligent design by anything resembling a loving, intelligent and maximally good god. Such evil could not exist in a universe created by such a god. Evil exists, therefore a maximally good, all-knowing, all-loving god does not.
Illustrated by Catherine Webber-Hounslow.
Available in Hardcover, Paperback or ebook for Kindle
Illustrated by Catherine Webber-Hounslow.






No comments :
Post a Comment
Obscene, threatening or obnoxious messages, preaching, abuse and spam will be removed, as will anything by known Internet trolls and stalkers, by known sock-puppet accounts and anything not connected with the post,
A claim made without evidence can be dismissed without evidence. Remember: your opinion is not an established fact unless corroborated.