Readers may recall that I wrote recently about how the process of photosynthesis, on which almost all life on Earth ultimately depends, involves an enzyme known as RuBisCo, and why it would be a major embarrassment to intelligent [sic] design creationists, if only they understood it. I also wrote about it in my popular book, The Unintelligent Designer: Refuting the Intelligent Design Hoax as an example of the sort of prolific waste that refutes the notion of intelligent design.
But it just got a lot worse for creationism because scientists have discovered that a soil bacterium, Kitasatospora setae has an enzyme which does the same job, only very much faster, which begs the question for intelligent [sic] design creationists, why didn't their putative intelligent designer give this enzyme to all photosynthesising organisms, so saving waste and making more efficient use of the available CO2? Did it deliberately give them all a less efficient process?
The discovery was made by an international team of scientists from the Department of Energy’s SLAC National Accelerator Laboratory, Stanford University, USA, Max Planck Institute for Terrestrial Microbiology in Germany, DOE’s Joint Genome Institute (JGI) and the University of Concepción in Chile. They have published their findings, open access in the journal ACS Central Science, this week.
As the Stamford National Accelerator Laboratory (SLAC) news release explains:
Plants rely on a process called carbon fixation – turning carbon dioxide from the air into carbon-rich biomolecules – for their very existence. That’s the whole point of photosynthesis, and a cornerstone of the vast interlocking system that cycles carbon through plants, animals, microbes and the atmosphere to sustain life on Earth.More information is provided in the open access paper in ACS Central Science:
But the carbon fixing champs are not plants, but soil bacteria. Some bacterial enzymes carry out a key step in carbon fixation 20 times faster than plant enzymes do, and figuring out how they do this could help scientists develop forms of artificial photosynthesis to convert the greenhouse gas into fuels, fertilizers, antibiotics and other products.
Now a team of researchers … has discovered how a bacterial enzyme – a molecular machine that facilitates chemical reactions – revs up to perform this feat.This bacterial enzyme is the most efficient carbon fixer that we know of, and we came up with a neat explanation of what it can do. Some of the enzymes in this family act slowly but in a very specific way to produce just one product. Others are much faster and can craft chemical building blocks for all sorts of products. Now that we know the mechanism, we can engineer enzymes that combine the best features of both approaches and do a very fast job with all sorts of starting materials.
Professor Soichi Wakatsuki, co-leader of the study
Biosciences Division
SLAC National Accelerator Laboratory, Menlo Park, California, USA And Structural Biology Department
Stanford University, Stanford, California, USA.
Rather than grabbing carbon dioxide molecules and attaching them to biomolecules one at a time, they found, this enzyme consists of pairs of molecules that work in sync, like the hands of a juggler who simultaneously tosses and catches balls, to get the job done faster. One member of each enzyme pair opens wide to catch a set of reaction ingredients while the other closes over its captured ingredients and carries out the carbon-fixing reaction; then, they switch roles in a continual cycle.
A single spot of molecular “glue” holds each pair of enzymatic hands together so they can alternate opening and closing in a coordinated way, the team discovered, while a twisting motion helps hustle ingredients and finished products in and out of the pockets where the reactions take place. When both glue and twist are present, the carbon-fixing reaction goes 100 times faster than without them.This animation shows two of the paired molecules (blue and white) within the ECR enzyme, which fixes carbon in soil microbes, in action. They work together, like the hands of a juggler who simultaneously tosses and catches balls, to get the job done faster. One member of each enzyme pair opens wide to catch a set of reaction ingredients (shown coming in from top and bottom) while the other closes over its captured ingredients and carries out the carbon-fixing reaction; then, they switch roles in a continual cycle. Scientists are trying to harness and improve these reactions for artificial photosynthesis to make a variety of products.
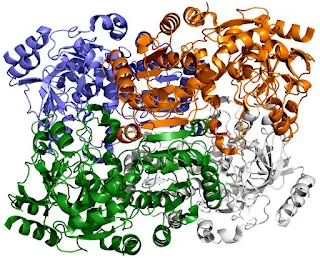
The enzyme the team studied is part of a family called enoyl-CoA carboxylases/reductases, or ECRs. It comes from soil bacteria called Kitasatospora setae, which in addition to their carbon-fixing skills can also produce antibiotics.This depiction of ECR, an enzyme found in soil bacteria, shows each of its four identical molecules in a different color. These molecules work together in pairs – blue with white and green with orange – to turn carbon dioxide from the microbe’s environment into biomolecules it needs to survive. A new study shows that a spot of molecular glue and a timely swing and twist allow these pairs to sync their motions and fix carbon 20 times faster than plant enzymes do during photosynthesis.
As important as photosynthesis is to life on Earth, [Tobias] Erb said, it isn’t very efficient. Like all things shaped by evolution over the eons, it’s only as good as it needs to be, the result of slowly building on previous developments but never inventing something entirely new from scratch.
What’s more, he said, the step in natural photosynthesis that fixes CO2 from the air, which relies on an enzyme called Rubisco, is a bottleneck that bogs the whole chain of photosynthetic reactions down. So using speedy ECR enzymes to carry out this step, and engineering them to go even faster, could bring a big boost in efficiency.
Meanwhile, Erb’s group in Germany and Associate Professor Esteban Vöhringer-Martinez’s group at the University of Concepción in Chile carried out detailed biochemical studies and extensive dynamic simulations to make sense of the structural data collected by Wakatsuki and his team.This twist is almost like a rachet that can push a finished product out or pull a new set of ingredients into the pocket where the reaction takes place.
If we can increase the rate of those sophisticated reactions to make new biomolecules, that would be a significant jump in the field.
Professor Soichi Wakatsuki
The simulations revealed that the opening and closing of the enzyme’s two parts don’t just involve molecular glue, but also twisting motions around the central axis of each enzyme pair, Wakatsuki said.
Together, the twisting and synchronization of the enzyme pairs allow them to fix carbon 100 times a second.
AbstractQuestion for intelligent [sic] design creationists then are:
Enoyl-CoA carboxylases/reductases (ECRs) are some of the most efficient CO2-fixing enzymes described to date. However, the molecular mechanisms underlying the extraordinary catalytic activity of ECRs on the level of the protein assembly remain elusive. Here we used a combination of ambient-temperature X-ray free electron laser (XFEL) and cryogenic synchrotron experiments to study the structural organization of the ECR from Kitasatospora setae. The K. setae ECR is a homotetramer that differentiates into a pair of dimers of open- and closed-form subunits in the catalytically active state. Using molecular dynamics simulations and structure-based mutagenesis, we show that catalysis is synchronized in the K. setae ECR across the pair of dimers. This conformational coupling of catalytic domains is conferred by individual amino acids to achieve high CO2-fixation rates. Our results provide unprecedented insights into the dynamic organization and synchronized inter- and intrasubunit communications of this remarkably efficient CO2-fixing enzyme during catalysis.
DeMirci, Hasan; Rao, Yashas; Stoffel, Gabriele M.; Vögeli, Bastian; Schell, Kristina; Gomez, Aharon; Batyuk, Alexander; Gati, Cornelius; Sierra, Raymond G.; Hunter, Mark S.; Dao, E. Han; Ciftci, Halil I.; Hayes, Brandon; Poitevin, Fredric; Li, Po-Nan; Kaur, Manat; Tono, Kensuke; Saez, David Adrian; Deutsch, Samuel; Yoshikuni, Yasuo; Grubmüller, Helmut; Erb, Tobias J.; Vöhringer-Martinez, Esteban; Wakatsuki, Soichi
Intersubunit Coupling Enables Fast CO2-Fixation by Reductive Carboxylases
ACS Central Science 2022; DOI: 10.1021/acscentsci.2c00057
Copyright: © 2022 The authors. Published by American Chemical Society
Open access
Reprinted under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International license (CC BY-NC-ND 4.0)
- If you believe this system was intelligently designed by the same designer you believe designed all living things, why didn't it provide all the others with this far more efficient system too?
- Why are scientists on the verge of a major breakthrough by improving on a design by your supposedly perfect designer?
- In what way is making your putative designer look like an incompetent fool for designing a proven bad system compared to how else the outcome could have been achieved, better than accepting that these systems are the result of a mindless, unintelligent natural process, like biologists claim?





No comments :
Post a Comment
Obscene, threatening or obnoxious messages, preaching, abuse and spam will be removed, as will anything by known Internet trolls and stalkers, by known sock-puppet accounts and anything not connected with the post,
A claim made without evidence can be dismissed without evidence. Remember: your opinion is not an established fact unless corroborated.