Discovery Illuminates How Sleeping Sickness Parasite Outsmarts Immune Response | Johns Hopkins | Bloomberg School of Public Health
Trypanosoma brucei is a blood-borne eukaryote parasite that should leave believers in an intelligent designer, open-mouthed in admiration for its inventive genius. Christian fundamentalist creationists of the white supremacist persuasion should also admire the racist that, through T. brucei, has managed to keep large parts of Africa technologically under-developed due to the difficult in maintaining herds of domestic animals where the vector of these parasites - the tsetse fly - is common.
As a vector, the tsetse fly is a triumph of malevolent design which I mentioned in my popular book, The Unintelligent Designer: Refuting the Intelligent Design Hoax, but it would have been all for nothing without the nasty little T. brucei to cause sleeping sickness in humans and the debilitating disease "nagana" in cattle.
What creationist admires of the divine malevolence they believe designs these things should now be marveling at is the sheer brilliance of the design by which it manages to evade the immune system, which they believe was created by the same designer god which now regards his design as a problem to be overcome oh parasites like T. brucei can continue making Africans and their cattle sick.
Trypanosoma brucie. Trypanosoma brucei is a protozoan parasite responsible for African trypanosomiasis, also known as "sleeping sickness" in humans and "nagana" in animals. It's a fascinating yet dangerous parasite because it has evolved numerous adaptations to survive and proliferate within its mammalian hosts. Here’s a breakdown of what makes T. brucei unique, particularly from an evolutionary perspective:This was discovered by researchers at the Johns Hopkins Bloomberg School of Public Health who have just published their findings in Nature and explained them in a Johns Hopkins Blomberg School of Health press release:
- Transmission and Life Cycle
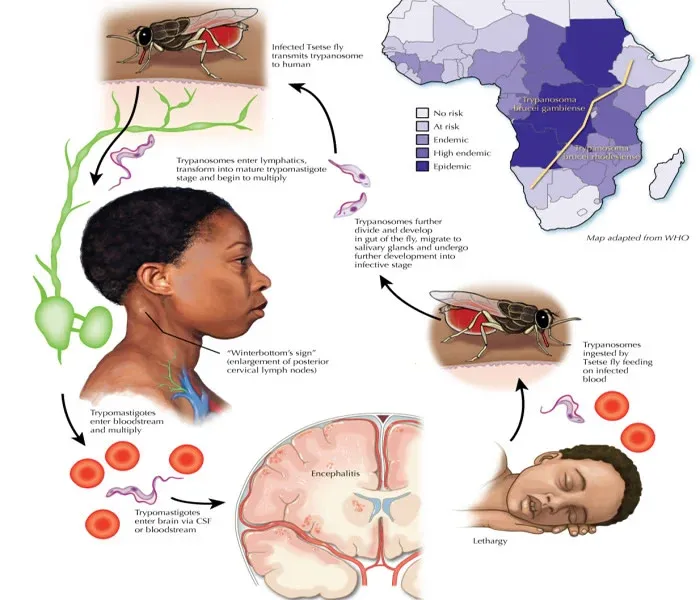
T. brucei is transmitted by the tsetse fly (Glossina species). When the fly bites a mammalian host, it injects the parasite in its infectious form, the metacyclic trypomastigote, into the bloodstream. T. brucei then multiplies in the blood and lymphatic system of the host, later invading the central nervous system, which results in the hallmark symptoms of sleeping sickness. Infected hosts can then pass the parasite back to other tsetse flies, which continues the transmission cycle.
- Evolution and Adaptations
The genus Trypanosoma has evolved over millions of years, believed to have diverged from other eukaryotic organisms more than 500 million years ago. Several key adaptations have allowed T. brucei to become such a successful parasite:
- Antigenic Variation: One of the most remarkable evolutionary traits of T. brucei is its ability to evade the host's immune system through antigenic variation. The parasite covers itself in a single variant surface glycoprotein (VSG) coat. When the host immune system starts to recognize and attack this VSG, the parasite switches to a different VSG gene, producing a new coat and avoiding immune detection. This constant switching of VSG genes allows T. brucei to survive in the host for prolonged periods.
- Kinetoplastid Features: T. brucei is part of the Kinetoplastida order, which has a unique structure called the kinetoplast, a dense region of DNA within the single mitochondrion of each cell. The kinetoplast contains multiple circular DNA molecules, providing insights into both the unique biology of trypanosomes and their evolutionary divergence from other eukaryotes.
- Adaptation to Varying Environments: T. brucei has evolved mechanisms to survive in vastly different environments: the bloodstream of vertebrates and the gut of tsetse flies. This requires significant metabolic flexibility. For instance, in the tsetse fly, the parasite relies on proline as an energy source, while in the mammalian bloodstream, it shifts to glucose metabolism.
- Evolutionary Origins and Speciation
There are several subspecies of T. brucei, with each adapted to specific hosts or regions in Africa:
- T. brucei gambiense: Causes chronic human African trypanosomiasis in West and Central Africa.
- T. brucei rhodesiense: Causes acute human African trypanosomiasis in East Africa.
- T. brucei brucei: Primarily infects animals and is not pathogenic to humans.
These subspecies have diverged due to geographic and host-specific pressures, adapting their virulence and infection strategies to their particular niches. Genetic analyses suggest that these subspecies split from a common ancestor due to ecological pressures and host availability.
- Impact and Co-Evolution with Hosts
The presence of T. brucei has also influenced the evolution of mammals in Africa. For example, certain populations of African humans and animals have developed partial resistance or tolerance to the parasite, such as the trypanolytic factor in some human populations. In turn, T. brucei continues to adapt, creating a classic example of host-parasite co-evolution.- Medical and Ecological Significance
Beyond its evolutionary interest, T. brucei remains a major public health issue in parts of sub-Saharan Africa, with human and animal trypanosomiasis affecting millions. Efforts to control the tsetse fly population, develop better treatments, and understand the parasite’s biology continue to be important in combating this disease.
Discovery Illuminates How Sleeping Sickness Parasite Outsmarts Immune Response
Parasite spread by tsetse flies persists in hosts by hiding in tissues and evading antibodies
A new study led by researchers at the Johns Hopkins Bloomberg School of Public Health sheds light on how the blood-borne parasite that causes African sleeping sickness in humans and related diseases in cattle and other animals establishes long-term infections in hosts. Using a mouse model, the researchers showed that Trypanosoma brucei essentially plays a game of hide-and-seek by setting up shop in its hosts’ tissues, allowing it to constantly change its protective surface coat and evade antibodies.
The discovery, reported October 30 in Nature, could potentially pave the way for understanding the immune response to other pathogens.
African sleeping sickness—also known as human African trypanosomiasis—is a neglected tropical disease that is usually fatal to humans if untreated. While treatment campaigns and tsetse fly control efforts have helped control human African trypanosomiasis, T. bruceiremains a major problem for African farmers, killing an estimated three million cattle per year.
The T. brucei parasite constantly changes a surface coat made up of millions of copies of a single protein—the variant surface glycoprotein (VSG). Once one VSG has been recognized by a host’s antibody response, the parasite has already “switched” to a new one, which the immune system hasn’t spotted yet. By changing which of these variant genes is active, the parasite can alter its appearance enough to evade its host’s immune response for long periods.
The T. bruceiparasite, after entering the bloodstream via a tsetse fly bite, also infects tissues outside the bloodstream. The function of this “extravascular” home for the parasite had previously been unclear. To get a fuller understanding of how T. brucei evades the immune system, the researchers used a customized RNA sequencing method to catalog the distinct VSG types that appeared over time during T. bruceiinfections in mice.
The researchers discovered that the vast majority of VSGs arose in tissues rather than in the bloodstream. They also found that parasite clearance by the immune system is slower in tissues. Their findings suggest that moving from the bloodstream into extravascular spaces gives T. brucei important “breathing room” in which to generate enough VSG variants to survive long-term in hosts, ensuring continued transmission.
This work sets the stage for a new way of thinking about immune evasion in T. brucei infection and potentially other chronic infections.
Associate Professor Monica Mugnier, PhD, senior author
Department of Molecular Microbiology and Immunology
Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
The study’s first author, Alexander Beaver, PhD, was a doctoral candidate in the Mugnier laboratory at the time of the study.
Transmitted by the tsetse fly, T. bruceiparasites are found in many parts of sub-Saharan Africa. Many wild mammals in Africa harbor the parasite without showing signs of disease, and thus serve as reservoirs. In humans, and in livestock such as cattle, sheep, and goats, T. bruceiinfection can cause a chronic disease that, in later stages, involves severe lethargy and other neurological symptoms.
The parasite enters its mammalian hosts via the bloodstream during a tsetse fly bite, and usually exits the same way. Why T. bruceispreads from the blood into extravascular spaces has been an unresolved question. In the study, Mugnier and her team used their own VSG-targeted RNA-sequencing method to record the expression of different VSGs over time in T. bruceiparasites recovered from both the blood and tissues of infected mice.
They showed that once an infection had been established for more than a week or so, the number of detectable VSGs in mouse tissues was, on average, several times higher than the number in blood. Thus, the bulk of VSG diversity—the key determinant of the pathogen’s ability to evade the immune response—was seen in tissues, not blood. This was the case whether the mice were infected by needle or by tsetse fly bite.
When the researchers tracked specific VSGs from initial infection, they found that immune clearance of parasites bearing these VSGs occurred significantly later in tissues than in blood. Similarly, when they used mice engineered so that tissue clearance of the parasite was further delayed, tissue VSG diversity was correspondingly greater.
The results overall suggest that T. bruceiuses tissues as relatively protected spaces in which it can survive longer, generating a greater diversity of variants, and potentially re-seeding the bloodstream with these variants—faster than the immune system can keep up.
Tissues were once thought to be incidental sites of infection for T. brucei, but it now appears that they might play an important role in maintaining a long-term infection for this parasite, while the bloodstream may serve more as a highway system for movement between tissues and for eventual transmission back into the tsetse fly
T. brucei infection could turn out to be a useful model for understanding why extravascular spaces are less efficient at pathogen clearance and how some pathogens exploit that.
Associate Professor Monica Mugnier, PhD
This new model suggests in turn, she adds, that disrupting T. brucei’s ability to spread from blood to tissues could be enough to allow the immune system to catch up and terminate infection, and thus could be a valuable new treatment strategy.
Mugnier and colleagues also suspect that this exploitation of extravascular spaces may be a tactic used by other pathogens—the Lyme disease bacterium, for example—to establish chronic infections.
“Tissue spaces are reservoirs of antigenic diversity for Trypanosoma brucei” was co-authored by Alexander Beaver, Zhibek Keneskhanova, Raul Cosentino, Brian Weiss, Erick Awuoche, Gretchen Smallenberger, Gracyn Buenconsejo, Nathan Crilly, Jaclyn Smith, Jill M.C. Hakim, Bailin Zhang, Bryce Bobb, Filipa Rijo-Ferreira, Luisa Figueiredo, Serap Aksoy, T. Nicolai Siegel, and Monica Mugnier.
AbstractNow, I expect creationists will dutifully trot out their traditional excuses in the form of the biologically nonsensical gobbledygook obligingly provided by Michael J Behe and his Deception Institute - 'Genetic entropy' and 'Devolution', but none of them will be able to explain how genetic entropy increases in a species gene pool without loss of genetic function and how deleterious genes increase in a species gene pool. And none of them will be able to explain why a mutation which gives a parasite an improved ability to parasitise its host and reproduce in it, can be regarded as something other than evolution by natural selection.
The protozoan parasite Trypanosoma brucei evades clearance by the host immune system through antigenic variation of its dense variant surface glycoprotein (VSG) coat, periodically ‘switching’ expression of the VSG using a large genomic repertoire of VSG-encoding genes1,2,3,4,5,6. Recent studies of antigenic variation in vivo have focused near exclusively on parasites in the bloodstream6,7,8, but research has shown that many, if not most, parasites reside in the interstitial spaces of tissues9,10,11,12,13. We sought to explore the dynamics of antigenic variation in extravascular parasite populations using VSG-seq7, a high-throughput sequencing approach for profiling VSGs expressed in populations of T. brucei. Here we show that tissues, not the blood, are the primary reservoir of antigenic diversity during both needle- and tsetse bite-initiated T. brucei infections, with more than 75% of VSGs found exclusively within extravascular spaces. We found that this increased diversity is correlated with slower parasite clearance in tissue spaces. Together, these data support a model in which the slower immune response in extravascular spaces provides more time to generate the antigenic diversity needed to maintain a chronic infection. Our findings reveal the important role that extravascular spaces can have in pathogen diversification.
Beaver, A.K., Keneskhanova, Z., Cosentino, R.O. et al.
Tissue spaces are reservoirs of antigenic diversity for Trypanosoma brucei. Nature (2024). https://doi.org/10.1038/s41586-024-08151-z
© 2024 Springer Nature Ltd.
Reprinted under the terms of s60 of the Copyright, Designs and Patents Act 1988.
Sadly, unless creationists accept that these parasites have evolved, they are left with no alternative but to accept that their putative intelligent designer is a malevolent sadist.
The Malevolent Designer: Why Nature's God is Not Good
Illustrated by Catherine Webber-Hounslow.
The Unintelligent Designer: Refuting The Intelligent Design Hoax





No comments :
Post a Comment
Obscene, threatening or obnoxious messages, preaching, abuse and spam will be removed, as will anything by known Internet trolls and stalkers, by known sock-puppet accounts and anything not connected with the post,
A claim made without evidence can be dismissed without evidence. Remember: your opinion is not an established fact unless corroborated.