
New research by scientists from the University of Wisconsin-Madison together with colleagues from Yale, has shown that the difference between the human brain and that of the other apes and monkeys are actually small, but those small differences are responsible for the qualitative difference. As explained in the University of Wisconsin-Madison News release:
When comparing a chimpanzee to a human the differences seem huge — from their physical appearances down to the capabilities of their brains. But at the cellular and genetic level, at least in the prefrontal cortex, the similarities are many and the dissimilarities sparing.The team collected genetic information from more than 600,000 prefrontal cortex cells from tissue samples from humans, chimpanzees, macaques and marmosets. They then categorized the cells into different types and determined the differences between cells of similar type. They found the vast majority of cells were fairly comparable.
More detail is given in the abstract to their published paper in Science:Understanding the molecular differences that make the human brain distinct can help researchers study disruptions in its development. A new study, published recently in the journal Science by a team including University of Wisconsin–Madison neuroscience professor Andre Sousa, investigates the differences and similarities of cells in the prefrontal cortex — the frontmost region of the brain, an area that plays a central role in higher cognitive functions — between humans and non-human primates such as chimpanzees, Rhesus macaques and marmosets.We are profiling the dorsolateral prefrontal cortex because it is particularly interesting. This cortical area only exists in primates. It doesn’t exist in other species. It has been associated with several relevant functions in terms of high cognition, like working memory. It has also been implicated in several neuropsychiatric disorders. So, we decided to do this study to understand what is unique about humans in this brain region.
Most of the cells are actually very similar because these species are relatively close evolutionarily.
Our lab really wants to know what is unique about the human brain. Obviously from this study and our previous work, most of it is actually the same, at least among primates.
We want to know what happened after the evolutionary split between humans and other primates. The idea is you have a mutation in a gene or in several genes and those genes now have slightly different functions. But if these genes are relevant for brain development, for example, how many of a certain cell is produced, or how cells are connecting to other cells, how is it affecting the neuronal circuitry and their physiological properties? We want to understand how these differences lead to differences in the brain and then lead to differences we can observe in adults
We are able to do extraordinary things, right? We are studying life itself, the universe, and so much more. And this is really unique when you look around. If we have these unique abilities, it has to be something in the brain, right? There is something in the brain that allows us to do all of that and we are really interested in knowing what it is.
André M. M. Sousa, corresponding author
Waisman Center and Department of Neuroscience
School of Medicine and Public Health
University of Wisconsin-Madison, Madison, WI, USA.
The cellular differences between these species may illuminate steps in their evolution and how those differences can be implicated in disorders, such as autism and intellectual disabilities, seen in humans. Sousa, who studies the developmental biology of the brain at UW–Madison’s Waisman Center, decided to start by studying and categorizing the cells in the prefrontal cortex in partnership with the Yale University lab where he worked as a postdoctoral researcher.
Sousa and his collaborators found five cell types in the prefrontal cortex that were not present in all four of the species. They also found differences in the abundancies of certain cell types as well as diversity among similar cell populations across species. When comparing a chimpanzee to a human the differences seem huge — from their physical appearances down to the capabilities of their brains. But at the cellular and genetic level, at least in the prefrontal cortex, the similarities are many and the dissimilarities sparing.
[Sousa's] team included graduate students Ryan Risgaards and Zachary Gomez-Sanchez, research intern Danielle Schmidt, and undergraduate students Ashwin Debnath and Cade Hottman.
The slight differences the researchers found may be the beginning of determining some of those unique factors, and that information could lead to revelations about development and developmental disorders at a molecular level.
The study’s observations were made in the brains of adults, after much of the development is complete. This means that the differences may be occurring during the brain’s development. So, the researchers’ next step is to study samples from developing brains and extend their area of investigation past the prefrontal cortex to potentially find where and when these differences originate. The hope is that this information will lead to a more robust foundation to lay developmental disorder research on top of.
Structured AbstractCreationists will probably need to ignore the fact that there is not the slightest hint of the Theory of Evolution being inadequate to explain the observations. In fact, the observations are entirely consistent with the idea of common ancestry and the taxonomic classification of humans in relationship to other primates. If there were not already evidence of that from other sources, this finding would be that evidence.
INTRODUCTION
The dorsolateral prefrontal cortex (dlPFC) lies at the center of high-order cognition and complex social behaviors, which are highly derived traits in anthropoid primates—particularly in humans.
RATIONALE
The granular dlPFC represents an evolutionary specialization found only in anthropoid primates, and alterations in the molecular and cellular mechanisms underlying its intricate circuitry have been implicated in myriad neuropsychiatric diseases. However, little is known about the full repertoire of cell types in the primate dlPFC and how conserved these cell types are between human and other primate species.
RESULTS
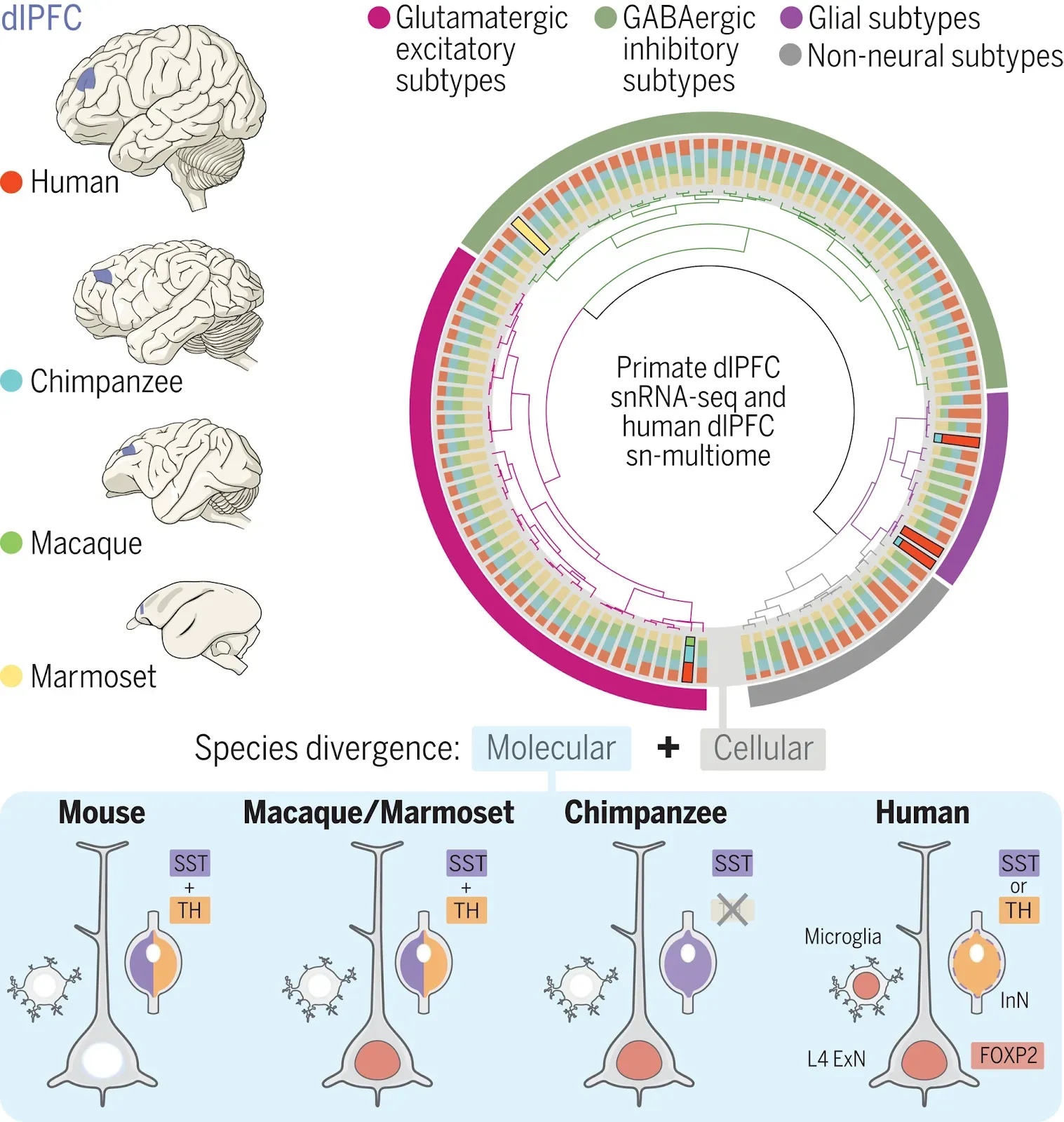
We generated single-nucleus transcriptome data profiling more than 600,000 nuclei from the dlPFC of adult humans, chimpanzees, rhesus macaques, and common marmosets, thus spanning major primate phylogenetic groups. To study regulatory mechanisms underlying human-specific divergence, we generated single-nucleus multiome data (snATAC-seq and snRNA-seq) profiling the human dlPFC. Through cell clustering, marker gene expression, and integration with published multimodal and multispecies data we defined three levels of hierarchically organized taxonomy of transcriptomically defined neuronal, glial, and non-neural cell types in the four species, including four major cell classes, 29 subclasses and 114 subtypes.
Most cell subtypes were conserved across the four species but we unraveled prominent species differences both at the molecular and cellular levels. We identified five cell subtypes detected in only a subset of species, including a layer (L) 2-3 intratelencephalic subtype absent in marmosets, an inhibitory neuron subtype exclusive to marmosets, and a microglial subtype detected only in humans. Cross-species comparisons of cell type proportions showed that L2-3 intratelencephalic neurons underwent substantial expansion in humans compared with other species as well as in Catarrhini as compared with marmosets. Gene expression entropy analysis revealed more transcriptomic heterogeneity among L2-3 intratelencephalic neurons in Catarrhini compared to marmosets. These results confirm and extend theories of primate cortical expansion.
Within homologous cell subtypes across species, we identified prominent molecular changes. These are characterized by loss of expression of tyrosine hydroxylase (TH), the rate-limiting enzyme in catecholamine (including dopamine) biosynthesis, in the inhibitory neurons of chimpanzees that are homologous to TH-expressing inhibitory neurons in the other species studied. Among TH-expressing homologous cell subtypes in humans, macaques, and marmosets, we identified a human-specific posttranscriptional switch between the neuropeptide SST and TH, and the human-specific expression of genes involved in dopaminergic function. Through transcriptomic comparisons across the four primate species and immunohistochemistry across 51 mammal species, we found that the neuropsychiatric risk gene FOXP2 exhibited human-specific expression in microglia and primate-specific expression in L4 excitatory neurons. By integrating chromatin accessibility and gene coexpression, we identified cis-regulatory elements regulating FOXP2 expression and constructed FOXP2 regulatory networks including downstream targets mirroring the cell type- and species-specific FOXP2 expression patterns.
CONCLUSION
We produced a transcriptomic catalog of the primate dlPFC cell types, complemented with epigenomic characterization in the human dlPFC. Our analyses delineated cell type homology and transcriptomic conservation across species and identified species divergence at the molecular and cellular levels, as well as potential epigenomic mechanisms underlying these differences. Shared and species-divergent features were implicated in biological pathways and neuropsychiatric diseases. Our data may serve as a resource for future studies on prefrontal cortex function and disease.
AbstractTranscriptomic taxonomy of the dlPFC in four anthropoid primates.
(Top left) Homologous regions of the dlPFC dissected for snRNA-seq and sn-multiome analyses. (Top right) 114 hierarchically organized (dendrogram) transcriptomically defined cell subtypes distributed across the four species (bar plots; same color code as in the top left panel), with species-specific variations highlighted. (Bottom) Notable molecular changes across species featured by species-specific FOXP2 expression and the human-specific posttranscriptional switching between SST and TH.
The granular dorsolateral prefrontal cortex (dlPFC) is an evolutionary specialization of primates that is centrally involved in cognition. We assessed more than 600,000 single-nucleus transcriptomes from adult human, chimpanzee, macaque, and marmoset dlPFC. Although most cell subtypes defined transcriptomically are conserved, we detected several that exist only in a subset of species as well as substantial species-specific molecular differences across homologous neuronal, glial, and non-neural subtypes. The latter are exemplified by human-specific switching between expression of the neuropeptide somatostatin and tyrosine hydroxylase, the rate-limiting enzyme in dopamine production in certain interneurons. The above molecular differences are also illustrated by expression of the neuropsychiatric risk gene FOXP2, which is human-specific in microglia and primate-specific in layer 4 granular neurons. We generated a comprehensive survey of the dlPFC cellular repertoire and its shared and divergent features in anthropoid primates.
Ma, Shaojie; Skarica, Mario; Li, Qian, et. al (2022) Molecular and cellular evolution of the primate dorsolateral prefrontal cortex
Science; 377(6614), eabo7257; DOI: 10.1126/science.abo7257
Copyright © 2022 The Authors
Published by American Association for the Advancement of Science
Reprinted with kind permission under license #5424790197487
The human brain might be larger and consequently capable of learning and assimilation of knowledge, but the differences are a matter of degree, not of fundamental structure or major genetic differences. The enhances abilities that human have compared to other primates is simply because certain structures in the brain contain more neurones. No magic is needed to explain that observable fact.


No comments :
Post a Comment
Obscene, threatening or obnoxious messages, preaching, abuse and spam will be removed, as will anything by known Internet trolls and stalkers, by known sock-puppet accounts and anything not connected with the post,
A claim made without evidence can be dismissed without evidence. Remember: your opinion is not an established fact unless corroborated.