It's reassuring to think humans are evolution's ultimate destination – but research shows we may be an accident
In the creationist parodies of the Theory of Evolution, evolution is presented as a theory that organisms become more and more complex and at the apex of evolution are human beings, the most complex of all species.
Of course, the real TOE says no such thing and claims about aims and purpose, with humans as the intended outcome, are childishly anthropocentric in the extreme. Nor is complexity the inevitable result of evolution since many species, especially parasites, have evolved with a loss of complexity and genetic information. But in general, ignoring parasites, much of evolution does include an increase in complexity, though not always an increase in genetic information. Many salamanders, such as the axolotl, have a much larger genome than humans and even the Christmas tree has a genome 7 times larger than the human genome.
The most complex organism, genetically speaking, is in fact a relatively simple, single-celled amoeba, Amoeba dubia, with a genome 60 times larger than the human genome.
Which species have the largest genomes and how do they compare to the human genome for size? The size of an organism's genome can vary significantly across different species, and some organisms indeed have much larger genomes than humans. The size of a genome is typically measured in base pairs (bp). The human genome contains approximately 3.2 billion base pairs. Some species with notably large genomes include:It's important to note that genome size doesn't necessarily correlate with the complexity or the number of genes in an organism. For example, while the human genome is much smaller than some of the organisms listed above, it is more complex and contains a higher number of protein-coding genes.
- Amoeba dubia: This single-celled amoeba has one of the largest genomes known, with an estimated size of around 200 billion base pairs. This is more than 60 times larger than the human genome.
- Paris japonica: This is a flowering plant native to Japan and has one of the largest known genomes among plants, estimated to be around 150 billion base pairs.
- Axolotl (Ambystoma mexicanum): The axolotl is a type of salamander that has a genome estimated to be around 32 billion base pairs, which is about 10 times larger than the human genome.
- Marbled lungfish (Protopterus aethiopicus): Lungfish, a type of fish, have large genomes, with some estimates suggesting sizes of around 130 billion base pairs.
The size of an organism's genome can be influenced by various factors, including the presence of repetitive sequences, non-coding DNA regions, and the evolutionary history of the species. The study of genome size and its significance in different organisms is a topic of ongoing research in the field of genomics.
Do you have any information on how these specific examples acquired such large genomes?
The acquisition of large genomes in various species, including the examples mentioned, is a complex and ongoing area of research in evolutionary biology and genomics. There isn't a single explanation for why certain species have evolved such large genomes, but scientists have proposed several mechanisms and factors that may contribute to the expansion of genome size in different lineages:
- Transposable Elements: Transposable elements, also known as "jumping genes," are sequences of DNA that can move around within a genome. They can replicate and insert themselves in various locations, sometimes leading to genome expansion over time. Some organisms with large genomes, like amoebas, may have accumulated a significant number of transposable elements.
- Gene Duplication: Gene duplication events can lead to the expansion of certain gene families within a genome. Over time, these duplicated genes can evolve new functions or become non-functional, contributing to genome size.
- Repetitive Sequences: Large genomes often contain a substantial portion of repetitive DNA sequences. These repetitive sequences can result from various processes, including transposable elements and duplication events. Repetitive sequences may not have specific functions in the organism's biology but can still make up a significant portion of the genome.
- Non-Coding DNA: Not all DNA in an organism's genome codes for proteins. Non-coding DNA, including regulatory regions, introns, and intergenic regions, can also contribute to genome size. The role of non-coding DNA in gene regulation and genome function is an active area of research.
- Evolutionary History: The evolutionary history of a species can play a role in genome size. Some lineages may have experienced bursts of genome expansion followed by periods of stability or reduction.
- Adaptation: In some cases, large genomes may confer certain advantages, such as increased genetic diversity or the ability to adapt to a wide range of environmental conditions. This can lead to the maintenance or expansion of genome size through natural selection.
It's important to note that genome size can vary widely even among closely related species, suggesting that multiple factors are at play in genome size evolution. Additionally, the significance and function of many non-coding regions and repetitive elements in large genomes are still areas of active research.
The specific mechanisms underlying the acquisition of large genomes in the examples you mentioned, such as Amoeba dubia, Paris japonica, Axolotl, and Marbled lungfish, would require detailed genomic and evolutionary studies to uncover. Researchers continue to investigate these and other species to gain insights into the factors driving genome size variation in the natural world.
There is still the question of how an increase in complexity occurs. Creationists, and even those theists who accept the evidence for evolution but want to find a place for their god in the process, like to assume that humans are more complex on purpose because their god had us in mind all along and set evolution going with us as the final product. Of course, the appearance of design is no indicator of conscious design because a mindless natural process such as natural selection can mimic the design process.
But complexity can also increase 'accidentally' by, for example, accidental gene duplication then repurposing under natural selection.
Two of the authors of the paper on bird evolution and hos complexity doesn't mean increased biodiversity, which was the subject of my last blog post, have published an article in The Conversation explaining this subtle but important distinction for understanding the process of evolution. Their article is reprinted here under a Creative Commons licence, reformatted for stylistic consistency:

It’s reassuring to think humans are evolution’s ultimate destination – but research shows we may be an accident
The Cambrian explosion, about 530 million years ago, was when most of the major groups of animals first appear in the fossil record.
Credit: canbedone/Shutterstock
Matthew Wills, University of Bath and Marcello Ruta, University of Lincoln
Depending upon how you do the counting, there are around 9 million species on Earth, from the simplest single-celled organisms to humans.
It’s reassuring to imagine that complex bodies and brains like ours are the inevitable consequence of evolution, as if evolution had a goal. Unfortunately for human egos, a recent study comparing over a thousand mammals – the group we belong to – painted a less gratifying picture.
Evolutionary biologists in the late 18th century, including Jean-Baptiste Lamarck, reasoned that life must have an innate tendency to evolve into ever more complex forms, and believed this reflected God’s design. However, by the mid-19th century, Charles Darwin showed that natural selection has no direction, and will sometimes make organisms simpler.
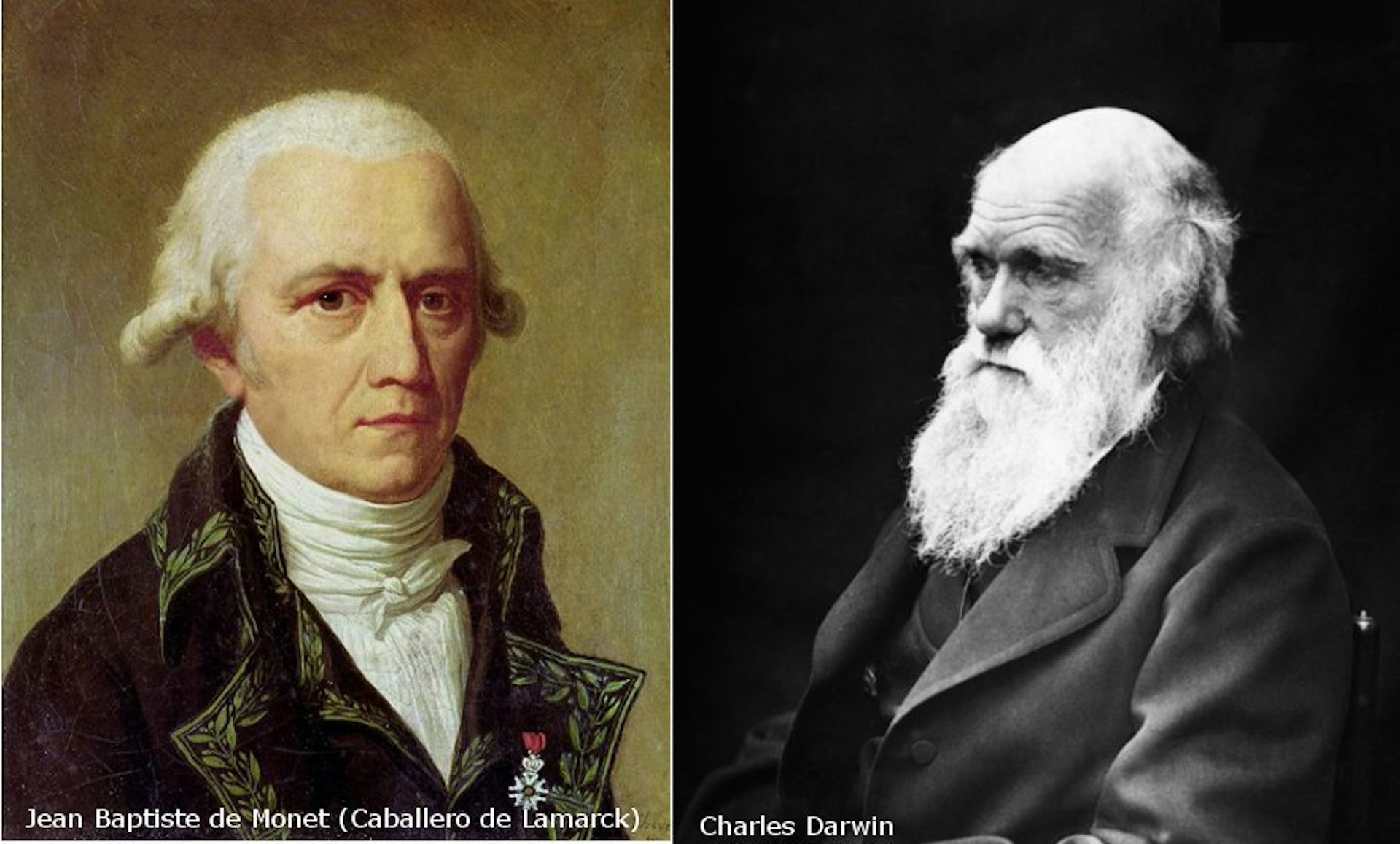
Wikimedia Commons
Because most organisms are still very simple, one possibility is that maximum complexity has increased “accidentally”, like the diffusion of a drop of ink in a glass of water. If true, this could be a blow to our human sense of significance as the most complex organisms.
Another theory is that increasing complexity is driven, on average, by natural selection. Sometimes selection acts on many, independent branches of the tree of life in a similar way and in parallel. This can produce similar effects in many of those branches and is known as a driven trend.
While driven trends need not imply divine purpose, they at least suggest that complexity was mostly an improvement, which is reassuring for us humans.
So which pattern is the most common in the evolution of complexity: accidental diffusion or driven trend?
Most changes and mutations are bad, and these variants are usually weeded out through a process called stabilising selection, which acts to maintain the status quo. But if most mutations make things function less well, doesn’t this make it very difficult for evolutionary novelties to arise?
In fact, evolution often operates on multiple copies of things. For example, a single gene might be duplicated within the same organism.
Provided one copy maintains its original function, the other copy can accumulate mutations without putting its bearer at an immediate disadvantage. These mutated copies are usually edited out over time, but occasionally they acquire a new function that gives an advantage.
Even more remarkably, whole genomes – every single gene in an organism – can be duplicated in one generation. Under these circumstances, there are many chances that copies of some genes will acquire a new function.
For example, sturgeons and paddle fishes underwent a whole genome duplication 250 million years ago, and this may explain how they survived the biggest ever mass extinction that wiped out 96% of other marine species.

Millipedes have lots of pairs of legs that are essentially identical. Shrimps have fewer pairs of legs but with many different functions.
Shrimps, by contrast have many different types of legs modified for feeding, walking, swimming and brooding eggs. A biological principle called the zero force evolutionary law states that these copies will tend to become less similar by accidental diffusion alone, unless stabilising selection acts to keep the status quo. Of course, natural selection may also act to make the copies less similar if this has an advantage.
Our paper shows that increasing complexity in mammals has both diffusive and driven aspects. Rather than marching towards greater complexity, mammals evolved in lots of different directions, with only some lineages pushing the upper bounds of complexity.
Surely nature selects for complexity just a bit?
Unfortunately, there is little research addressing this question. One of the few published studies demonstrates that crustaceans (crabs, lobsters, shrimps and their relatives) evolved with a driven trend for increasing complexity over the last half a billion years.
Like crustaceans, and all vertebrates, we have bodies made of repeating blocks of tissue (called somites). These are most visible in our vertebral column (or spine) and ribs, and in the six-pack of a lean athlete. Across mammals, the number of vertebrae (the bones that make up the spine) varies and they are shaped to do different jobs in the neck, thorax, back, sacrum and tail.
Counting the number of bones in different regions can quantify one aspect of complexity across all mammals. In our study sampling over a thousand mammal species, many groups – including whales, bats, rodents, carnivores and, our own group, the primates – independently evolved complex vertebral columns. This suggests higher complexity can be a winning formula, and that selection is driving this in multiple branches of the mammal tree.
However, many other branches have a low plateau in complexity or even become simpler. Elephants, rhinos, sloths, manatees, armadillos, golden moles and platypuses all thrived despite the fact they have relatively simple vertebral columns. The direction of evolution all depends on context.
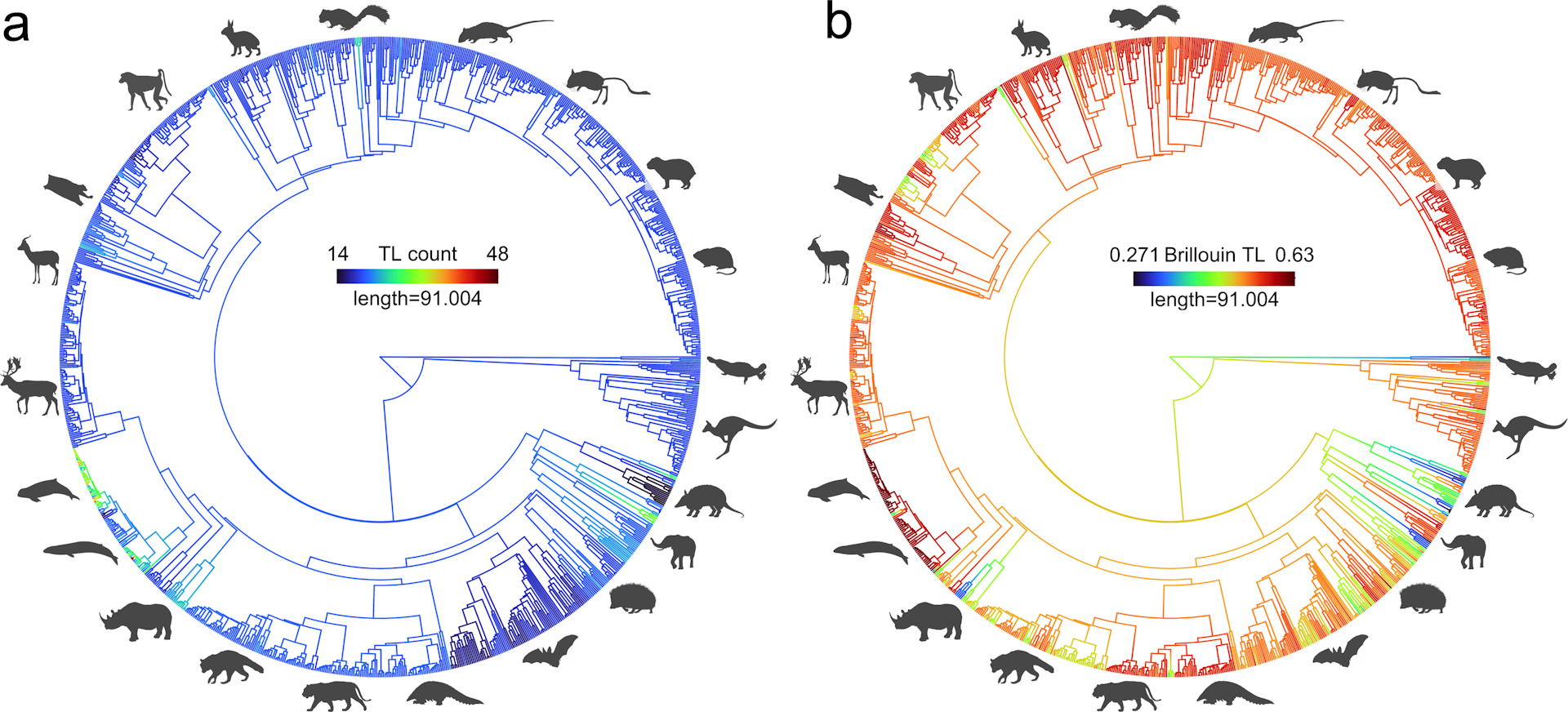
In a study of 1,136 mammal species, we find a driven trend for increasing complexity in multiple parallel lineages. Left hand panel is the number of vertebrae. Right hand panel is our index of complexity. Blue lines are lower values, while red lines are higher values.
Li et al; Nature Ecology & Evolution (2023), CC BY-ND
Matthew Wills, Professor of Evolutionary Palaeobiology at the Milner Centre for Evolution, University of Bath and Marcello Ruta, Senior Lecturer, Life Sciences, University of Lincoln
This article is republished from The Conversation under a Creative Commons license. Read the original article.
AbstractThere is, of course, no doubt here that the natural process of evolution is responsible for change in complexity both over time and across taxons, being responsible for what creationists like to call 'macroevolution'; the only question is the precise mechanisms involved.
Complexity, defined as the number of parts and their degree of differentiation, is a poorly explored aspect of macroevolutionary dynamics. The maximum anatomical complexity of organisms has undoubtedly increased through evolutionary time. However, it is unclear whether this increase is a purely diffusive process or whether it is at least partly driven, occurring in parallel in most or many lineages and with increases in the minima as well as the means. Highly differentiated and serially repeated structures, such as vertebrae, are useful systems with which to investigate these patterns. We focus on the serial differentiation of the vertebral column in 1,136 extant mammal species, using two indices that quantify complexity as the numerical richness and proportional distribution of vertebrae across presacral regions and a third expressing the ratio between thoracic and lumbar vertebrae. We address three questions. First, we ask whether the distribution of complexity values in major mammal groups is similar or whether clades have specific signatures associated with their ecology. Second, we ask whether changes in complexity throughout the phylogeny are biased towards increases and whether there is evidence of driven trends. Third, we ask whether evolutionary shifts in complexity depart from a uniform Brownian motion model. Vertebral counts, but not complexity indices, differ significantly between major groups and exhibit greater within-group variation than recognized hitherto. We find strong evidence of a trend towards increasing complexity, where higher values propagate further increases in descendant lineages. Several increases are inferred to have coincided with major ecological or environmental shifts. We find support for multiple-rate models of evolution for all complexity metrics, suggesting that increases in complexity occurred in stepwise shifts, with evidence for widespread episodes of recent rapid divergence. Different subclades evolve more complex vertebral columns in different configurations and probably under different selective pressures and constraints, with widespread convergence on the same formulae. Further work should therefore focus on the ecological relevance of differences in complexity and a more detailed understanding of historical patterns.
Li, Y., Brinkworth, A., Green, E. et al. Divergent vertebral formulae shape the evolution of axial complexity in mammals. Nat Ecol Evol 7, 367–381 (2023). https://doi.org/10.1038/s41559-023-01982-5
Copyright: © 2023 The authors.
Published by Springer Nature Ltd. Open access
Reprinted under a Creative Commons Attribution 4.0 International license (CC BY 4.0)
And of course, as it routine with biological research, and scientific research in general, the findings utterly refute creationist superstitions and in this case the parodies of science the cult leaders routinely fool their victims with, in order to maintain their income streams and in pursuit of extremist political objectives which require people to be misinformed and ignorant of real-world science.



No comments :
Post a Comment
Obscene, threatening or obnoxious messages, preaching, abuse and spam will be removed, as will anything by known Internet trolls and stalkers, by known sock-puppet accounts and anything not connected with the post,
A claim made without evidence can be dismissed without evidence. Remember: your opinion is not an established fact unless corroborated.