An evolutionary mystery 125 million years in the making | Cold Spring Harbor Laboratory
In an example of one of those lovely gaps in the record of the evolution of a species into which creationists try to shoehorn their ever-shrinking and increasingly homeless little god, there is something about the evolution of tomatoes and Arabidopsis thaliana that scientists can't yet explain.
But the problem for creationists is that this gap is somewhere in the evolutionary history of these plants that occurred almost 125 million years before creationism’s god decided to create a small flat planet with a dome over it to keep the water above the sky out, centred on the Middle East, in what creationists like to call 'Creation Week'.
The problem comes from the fact that what creationists think is a science and history text book was written by ignorant people who knew nothing of the world outside their small part of it and who had no idea about the history of the planet or of life on it, so they wrote an imaginative story to fill the gap in their knowledge and understanding, and, quite understandably, got almost every aspect of it complete wrong.
And of course, they would never have imagined that one day someone almost as ignorant as they were, would gather their tales into a book and declare it to be the inerrant word of a god - an idea that would be hilarious if it wasn't taken seriously by adults who can become dangerously violent when their superstition is questions.
The mystery that Cold Spring Harbor Laboratory (CSHL) biologists have uncovered is that sometime during the last 125 million years, tomatoes and Arabidopsis thaliana plants experienced an extreme genetic makeover. Just what happened remains unclear. But the mystery surrounds CLV3, a gene key to healthy plant growth and development.
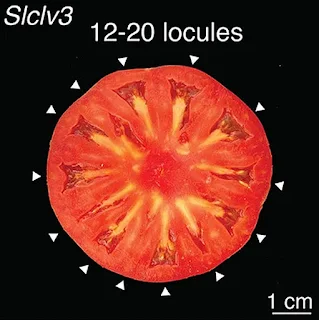
CLV3 controls the growth of fruit in these plants and, if uncontrolled will result in large, even gigantic, fruits, so there is an evolutionary trade-off between a few large fruits and lots of smaller fruits. The mystery is just how and why this balance was achieved differently in two distantly-related plants.
As the CSHL press release explains:
Plant genomics has come a long way since Cold Spring Harbor Laboratory (CSHL) helped sequence the first plant genome. But engineering the perfect crop is still, in many ways, a game of chance. Making the same DNA mutation in two different plants doesn’t always give us the crop traits we want. The question is why not? CSHL plant biologists just dug up a reason.More information is given in the team's paper in PLOS Genetics:
CSHL Professor and HHMI Investigator Zachary Lippman and his team discovered that tomato and Arabidopsis thaliana plants can use very different regulatory systems to control the same exact gene. Incredibly, they linked this behavior to extreme genetic makeovers that occurred over 125 million years of evolution.
The scientists used genome editing to create over 70 mutant strains of tomato and Arabidopsis thaliana plants. Each mutation deleted a piece of regulatory DNA around a gene known as CLV3. They then analyzed the effect each mutation had on plant growth and development. When the DNA keeping CLV3 in check was mutated too much, fruit growth exploded. Danielle Ciren, a recent CSHL School of Biological Sciences graduate who led this study, explains:CLV3 helps plants develop normally. If it wasn’t turned on at the exact time that it is, then plants would look very different. All the fruits would be ginormous and not ideal. You have to balance growth and yield. If a plant has giant tomatoes but only two, is that as beneficial as a lower yield? There’s no simple solution. You’re always sacrificing something when you’re trying to get something improved.
Danielle Ciren, lead author
Cold Spring Harbor Laboratory
School of Biological Sciences
Cold Spring Harbor, New York, USA.For tomatoes, engineering mutations near the beginning but not the end of the CLV3 gene dramatically affected fruit size. For Arabidopsis, areas around both parts of the gene needed to be disrupted. This indicates something happened over the last 125 million years that made the plants evolve differently. Exactly what occurred remains a mystery. Ciren explains:Mutations in the CLV3gene can dramatically increase fruit size, as seen in tomatoes (top row) and Arabidopsis thaliana (bottom row).What is certain is that genetic regulation is not uniform between plant species. Unearthing these genetic differences could help make crop genome engineering more predictable. And that would be a big win not just for science but for farmers and plant breeders across the globe.You can’t go back to the common ancestor because they don’t exist anymore. So it’s hard to say what was the original state and how have things been mixed up. The most simple explanation is that there’s a regulatory element that’s conserved in some capacity, and it’s been altered in subtle ways. It is a bit unexpected.
Danielle Ciren
AbstractSo, the mystery in the evolutionary history of these two plants is how and why did separate ways of regulating the activity of the 'growth' gene, CLV3 evolve differently. For a creationist, there is not only the problem of this all happening so long before Earth existed according to their superstition, but the fact that, if we assume design, it is evidence of the Heath-Robinson machine creationism’s putative designer keeps designing, adding a new layer of complexity to make an earlier 'solution' work and the fact that it designed two different ways of doing the same thing from the same starting point.
A striking paradox is that genes with conserved protein sequence, function and expression pattern over deep time often exhibit extremely divergentcis-regulatory sequences. It remains unclear how such drasticcis-regulatory evolution across species allows preservation of gene function, and to what extent these differences influence howcis-regulatory variation arising within species impacts phenotypic change. Here, we investigated these questions using a plant stem cell regulator conserved in expression pattern and function over ~125 million years. Using in-vivo genome editing in two distantly related models, Arabidopsis thaliana (Arabidopsis) and Solanum lycopersicum (tomato), we generated over 70 deletion alleles in the upstream and downstream regions of the stem cell repressor gene CLAVATA3 (CLV3) and compared their individual and combined effects on a shared phenotype, the number of carpels that make fruits. We found that sequences upstream of tomato CLV3are highly sensitive to even small perturbations compared to its downstream region. In contrast, Arabidopsis CLV3 function is tolerant to severe disruptions both upstream and downstream of the coding sequence. Combining upstream and downstream deletions also revealed a different regulatory outcome. Whereas phenotypic enhancement from adding downstream mutations was predominantly weak and additive in tomato, mutating both regions of Arabidopsis CLV3caused substantial and synergistic effects, demonstrating distinct distribution and redundancy of functionalcis-regulatory sequences. Our results demonstrate remarkable malleability incis-regulatory structural organization of a deeply conserved plant stem cell regulator and suggest that major reconfiguration ofcis-regulatory sequence space is a common yet cryptic evolutionary force altering genotype-to-phenotype relationships from regulatory variation in conserved genes. Finally, our findings underscore the need for lineage-specific dissection of the spatial architecture ofcis-regulation to effectively engineer trait variation from conserved productivity genes in crops.
Author summary
We investigated the evolution ofcis-regulatory elements (CREs) and their interactions in the regulation of a plant stem cell regulator gene, CLAVATA3 (CLV3), in Arabidopsis and tomato. Despite diverging ~125 million years ago, the function and expression of CLV3is conserved in these species; however, cis-regulatory sequences upstream and downstream have drastically diverged, preventing identification of conserved non-coding sequences between them. We used CRISPR-Cas9 to engineer dozens of mutations within the cis-regulatory regions of Arabidopsis and tomato CLV3. In tomato, our results show that tomato CLV3function primarily relies on interactions among CREs in the 5’ non-coding region, unlike Arabidopsis CLV3, which depends on a more balanced distribution of functional CREs between the 5’ and 3’ regions. Therefore, despite a high degree of functional conservation, our study demonstrates divergent regulatory strategies between two distantly related CLV3orthologs, with substantial alterations in regulatory sequences, their spatial arrangement, and their relative effects on CLV3regulation. These results suggest that regulatory regions are not only extremely robust to mutagenesis, but also that the sequences underlying this robustness can be lineage-specific for conserved genes, due to the complex and often redundant interactions among CREs that ensure proper gene function amidst large-scale sequence turnover.
Introduction
Cis-regulatory control of gene expression is essential for the function of genes and the phenotypes they govern. Expression control depends on cis-regulatory elements (CREs), non-coding sequences of DNA bound by transcription factors that determine when, where, and to what level genes are expressed throughout growth and development. CREs can occur in many sequence contexts relative to the gene they regulate, including upstream (5’) and downstream (3’), within the gene itself (in UTRs, introns, and exons), and at distal sites far away. Molecular assays for chromatin accessibility, histone modifications, and transcription factor binding in many model organisms have been used to identify hundreds of thousands of putative CREs [1–7]. Numerous reporter studies have been used to predict the effect of CREs on expression, and more recently functional genomics studies leveraging massively-throughput assays have dissectedcis-regulatory control of gene expression at scale [2,8–13]. In contrast, much less is known about how perturbation of cis-regulatory sequence space impacts phenotypes in multi-cellular organisms, both within and between species. Empowered by genome editing, studies can now go beyond the limited number and diversity of natural cis-regulatory alleles to address previously intractable questions on the intricate organization and relationships of CREs underlying genotype-to-phenotype relationships. Compared to the strong selective pressures on protein-coding sequences,cis-regulatory regions and their modularly organized and often highly redundant CREs are much more tolerant to sequence change, and thus evolve more rapidly [14]. Additionally, transcription factor binding sites (TFBSs) are degenerate, and their organization in spacing, order, orientation, and number is often highly flexible [15]. As multiple sequence compositions can produce similar regulatory outcomes, identifying conserved non-coding sequences (CNSs) via conventional alignment strategies is difficult [16]. This problem is even more apparent over longer evolutionary time scales, ascis-regulatory divergence between orthologous genes often results in little to no sequence similarity [17,18]. Importantly, however, while natural variation in expression and phenotypes among related genotypes are most often associated withcis-regulatory change, affected genes typically do not deviate substantially from their original expression patterns and phenotypic consequences are largely limited to the tissues and organs in which the genes function [19–21]. Moreover, co-expression and gene knockout studies across widely divergent species have found that for many orthologous genes, expression programs and phenotypes controlled are broadly conserved [22–25]. Thus, how such deeply conserved genes can tolerate extremecis-regulatory change but still maintain shared functions over deep time is an open question. A prominent hypothesis is that despite overall sequence divergence, trans-factor identity along with the relative positioning and functional interactions among CREs are constrained to preserve control of orthologous genes over wide evolutionary distances [18,26]. Along with fundamental insights intocis-regulatory evolution, understanding such constraints, often termed as “grammar” [15], could accelerate efforts to transfer knowledge from model organisms to new species, especially for trait engineering. We addressed this question by taking advantage of Arabidopsis thaliana (Arabidopsis) and Solanum lycopersicum (tomato), two plant model systems separated by ~125 million years of evolution that offer equally powerful tools in genome editing and high throughput phenotyping [27]. We leveraged the CLAVATA3 (CLV3) gene, which encodes a functionally conserved signaling peptide that represses stem cell proliferation in a deeply conserved negative feedback loop with the stem cell promoting transcription factor gene WUSCHEL (WUS) [28]. Null mutations of CLV3 in both systems cause the same stem cell over-proliferation phenotypes, most evident in an increase in the number of floral organs, including the carpels that form seed compartments in fruits known as locules [28]. Using in vivo CRISPR-Cas9 genome editing of CLV3 in both species, we identify CREs, resolve their organization, and dissect their interactions. This comparative approach allowed direct assessment of genotype-to-phenotype relationships in an evolutionary context, revealing the dynamics ofcis-regulatory change of a conserved gene over deep time.
Ciren D, Zebell S, Lippman ZB (2024)
Extreme restructuring of cis-regulatory regions controlling a deeply conserved plant stem cell regulator.
PLoS Genet 20(3): e1011174. https://doi.org/10.1371/journal.pgen.1011174
Copyright: © 2024 The authors.
Published by PLoS. Open access.
Reprinted under a Creative Commons Attribution 4.0 International license (CC BY 4.0)
As evidence for intelligent design, this simply isn't; as evidence for unintelligent, utilitarian design, it's a perfect example.
And to make matters worse for creationists, there is not a shadow of doubt on the part of the scientists that this is the result of an evolutionary process with no sign that they are beginning to think that magic by a supernatural magician is a better explanation of the facts. The TOE remains, as it has been since 1859, the fundamental basis for understanding biology and every paper such as this one adds a little more support for what is now an unassailable scientific explanation.
The Unintelligent Designer: Refuting The Intelligent Design Hoax
What Makes You So Special? From The Big Bang To You






No comments :
Post a Comment
Obscene, threatening or obnoxious messages, preaching, abuse and spam will be removed, as will anything by known Internet trolls and stalkers, by known sock-puppet accounts and anything not connected with the post,
A claim made without evidence can be dismissed without evidence. Remember: your opinion is not an established fact unless corroborated.