How does a virus hijack insect sperm to control disease vectors and pests? | Penn State University
Wolbachia are a genus of bacteria that form a symbiotic relationship with about 50% of arthropod species, including insects and spiders but they can also manipulate the species for their own ends (in terms of breeding success). They are aided in this by a virus which is incorporated in their genome which has been shown to join forces with Wolbachia to ensure their own reproductive success in the form of females infected with the virus-bearing Wolbachia.
So completely have Wolbachia integrated with insects that one species of fruit fly has the entire Wolbachia genome incorporated into its own genome, making it, biologically, both bacterium and fruit fly.
One way Wolbachia ensure their own survival at the expense of the species of insect they infect is by making the sperm and egg incompatible if the female is not also a carrier of the right species of Wolbachia. And, to be on the safe side, two proteins produced by the virus break the sperm's DNA so any resulting embryo will be defective and will fail to develop. This ensures that only the females carrying the infection can breed, so increasing the Wolbachia and its virus in the gene pool.
The team who discovered this nasty little virus and how it acts selfishly, was co-led by Professor Seth R. Bordenstein, of the One Health Microbiome Center at Pennsylvania State University. They have published their findings in Science and described it in a Penn State News item.
But first, a little background on Wolbachia:
Tell me all about Wolbachia bacteria and what they do to male insects. Wolbachia is a genus of bacteria that infects a wide range of arthropods, including insects, spiders, and crustaceans. They are one of the most common and widespread symbionts in the world, estimated to infect up to 40% of all insect species. Wolbachia are intracellular bacteria, meaning they live inside the cells of their host organisms. Here are some key characteristics and effects of Wolbachia bacteria:Overall, Wolbachia bacteria have profound effects on the reproductive biology and ecology of their host organisms. Their ability to manipulate host reproduction has important implications for the evolution of host populations, as well as potential applications in the control of insect pests and the spread of vector-borne diseases.
- Reproductive Manipulation: One of the most notable features of Wolbachia is their ability to manipulate the reproductive biology of their hosts. They can induce several reproductive alterations, including:
- Cytoplasmic Incompatibility (CI): This is the most well-known effect of Wolbachia. When an infected male mates with an uninfected female or a female carrying a different strain of Wolbachia, the embryos fail to develop properly, leading to reduced viability or death. However, when both partners are infected with the same strain of Wolbachia, the embryos develop normally. This phenomenon gives infected females a reproductive advantage, as they can successfully reproduce with infected males while uninfected females cannot.
- Feminization: In some cases, Wolbachia can cause genetic males to develop as females or intersex individuals. This occurs because Wolbachia can alter the sex determination mechanisms of their hosts, leading to the development of female characteristics in genetic males.
- Male Killing: In certain species, Wolbachia selectively kills male embryos during development, skewing the sex ratio of the population towards females. This can enhance the reproductive success of infected females, as they have fewer competitors for resources.
- Parthenogenesis Induction: Wolbachia can induce parthenogenesis, a form of asexual reproduction where females produce offspring without mating with males. This allows infected females to reproduce even in the absence of males, further enhancing their reproductive success.
- Protection Against Pathogens: Wolbachia can also provide protection to their host organisms against certain pathogens. They do this by interfering with the replication and transmission of viruses such as dengue, Zika, and chikungunya viruses in mosquitoes. This has led to research into using Wolbachia-infected mosquitoes as a method of controlling the spread of mosquito-borne diseases.
- Vertical Transmission: Wolbachia are primarily transmitted vertically from mother to offspring. They are often found within the reproductive tissues of their hosts, ensuring their transmission to the next generation via the eggs.
- Diversity and Evolution: Wolbachia exhibit a high level of diversity, with multiple strains and variants infecting different host species. They can also undergo horizontal transfer between host species, contributing to their evolutionary dynamics.
Is anything known about the evolutionary origins of Wolbachia?
The evolutionary origins of Wolbachia bacteria are still a subject of debate among researchers, but several lines of evidence suggest that they have co-evolved with their arthropod hosts over millions of years. Some key points regarding the evolutionary origins of Wolbachia include:Overall, while the exact evolutionary origins of Wolbachia remain uncertain, evidence suggests that they have co-evolved with their arthropod hosts over long periods, shaping their biology and ecology in intricate ways. Further research into the evolutionary history of Wolbachia and their interactions with their hosts will continue to provide insights into their origins and evolutionary dynamics.
- Ancient Association: Wolbachia are believed to have ancient origins, with estimates suggesting they may have been present in arthropods for hundreds of millions of years. Fossil evidence of Wolbachia has been found in ancient insects preserved in amber, indicating that they have been associated with their hosts for a long time.
- Vertical Transmission: The primary mode of transmission of Wolbachia is vertical, from mother to offspring via the eggs. This mode of transmission suggests a long co-evolutionary history with their hosts, as it allows the bacteria to persist within host populations over generations.
- Diverse Host Range: Wolbachia infect a wide range of arthropod species, including insects, spiders, and crustaceans. The diversity of host species suggests that Wolbachia have had ample opportunity to co-evolve with different host lineages over evolutionary time.
- Phylogenetic Studies: Phylogenetic analyses of Wolbachia and their host organisms have provided insights into their evolutionary relationships. Studies have suggested that Wolbachia strains often cluster with their host species, indicating co-speciation and long-term association between the bacteria and their hosts.
- Horizontal Transfer: While vertical transmission is the primary mode of Wolbachia transmission, there is also evidence of horizontal transfer between host species. Horizontal transfer events may contribute to the spread of Wolbachia across different host lineages and influence their evolutionary dynamics.
A widespread bacteria called Wolbachia and a virus that it carries can cause sterility in male insects by hijacking their sperm, preventing them from fertilizing eggs of females that do not have the same combination of bacteria and virus. A new study led by microbiome researchers at Penn State has uncovered how this microbial combination manipulates sperm, which could lead to refined techniques to control populations of agricultural pests and insects that carry diseases like Zika and dengue to humans.Sadly, the team's research paper is behind a paywall:
The study published today (March 8) in Science.
When a male and female insect that both have Wolbachia mate, they successfully reproduce and pass on the bacteria. But when a male with Wolbachia mates with a female with no Wolbachia, the sperm are rendered lethal to the fertilized eggs, succumbing them to death. This system cunningly increases the proportion of offspring with Wolbachia and the virus in the next generation, because females with the bacteria successfully reproduce more frequently than females without.Wolbachia is the most widespread bacteria in animals and lives symbiotically within the reproductive tissues of about 50% of insect species, including some mosquitos and flies. Wolbachia has genes from a virus called prophage WO integrated into its genome. These genes — cifA and cifB — allow the bacteria to remarkably manipulate sperm and quickly spread through an insect population for their own good.
Seth R. Bordenstein, co-corresponding author
Professor of biology and entomology
Director of the One Health Microbiome Center
Departments of Biology and Entomology
Pennsylvania State University, PA, USA.
This system is being used in several ongoing pilot studies across the world to control insect pests and the harmful viral diseases they carry. For example, to control a population of agricultural or human pests that do not have the bacteria, scientists release males with Wolbachia in order to crash the population.
Wolbachia’s prophage WO genes code for proteins that interfere with normal development of sperm cells. These proteins impact a critical transformation during sperm development, when the sperm’s genome is repackaged and the sperm changes from a canoe-shape into a more refined needle-like shape.One of Wolbachia’s superpowers is that it blocks pathogenic RNA viruses such as Zika, dengue and chikungunya virus, so mosquitos with Wolbachia do not pass these viruses on to people when they bite. So, releases of both male and female mosquitos with Wolbachia in an area where it isn’t already present leads to replacement of the population with mosquitos that can no longer pass on a viral disease. The World Mosquito Program is now using Wolbachia to control viruses in 11 countries. With this study, we reveal the underlying mechanics of how this process works so we can fine-tune the technique to expand its scope in vector control measures.
Professor Seth R. Bordenstein.
This shape change is incredibly important to the success of sperm, and any interference can impact the sperm’s ability to travel in the female reproductive tract and successfully fertilize the egg. The transition is highly conserved in almost everything from insects to humans. Defects in this process can also cause male sterility in humans.
Assistant professor Rupinder Kaur, co-corresponding author
Departments of Biology and Entomology
Pennsylvania State University, PA, USA.
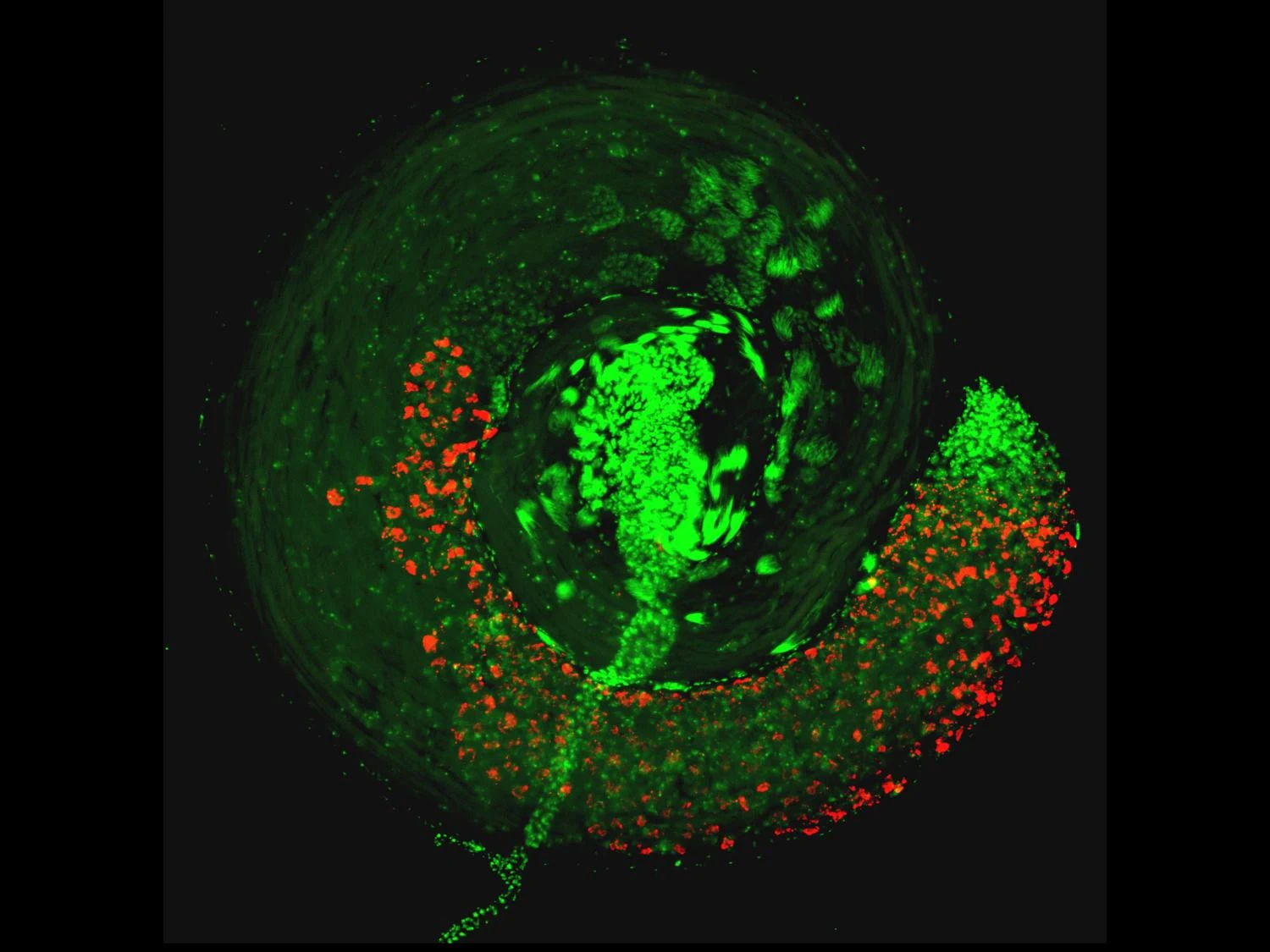
According to the researchers, sperm is particularly prone to DNA damage and repair during this transition. In this study, they found that sperm exposed to Wolbachia, or the Cif proteins alone, had an elevated level of DNA damage at this stage. The DNA damage, if not repaired in a timely fashion, can result in abnormal sperm genome packaging, male infertility and embryonic inviability.
These results confirmed the impact of Wolbachia and Cif proteins at this stage of sperm development, but we still wanted to know what was happening at earlier stages to trigger these changes. We conducted a series of tests to explore the structure and biochemical function of the Cif proteins and found that they can cleave messenger molecules called long non-coding RNA, which sets the stage to interfere with downstream development and function of the sperm.
Assistant professor Rupinder Kaur
The researchers used fruit flies with Wolbachia to test the potential link between the bacteria and long non-coding RNA. They found that Wolbachia — or the Cif proteins alone — reduced the amount of these RNAs. Additionally, mutant flies with reduced expression of these RNAs in conjunction with Wolbachia had elevated levels of embryonic inviability because it augmented the defective transition process of sperm development. So, Kaur explained, the virus proteins control sperm by depleting the long non-coding RNAs required for a normal sperm function.
Long non-coding RNAs do not make any proteins themselves, but they can have profound impacts on regulating the function of other genes required for sperm development. By altering this non-coding part of the genome, we found that Cif proteins start impacting sperm right from the earliest stages of development. Wolbachia’s prophage WO genes act like master puppeteers, manipulating sperm development in a way that allows their genes and the symbiotic bacteria to quickly spread through arthropod populations.
Professor Seth R. Bordenstein.
Because the process of sperm development looks similar across the animal kingdom, the researchers said that knowledge of this process could lend insight into sterility challenges in humans as well as inform new control methods of harmful insect populations.
In addition to Bordenstein and Kaur, the research team includes Angelina McGarry, research technologist II at Penn State; J. Dylan Shropshire, assistant professor at Lehigh University; and Brittany Leigh, a postdoctoral researcher at Vanderbilt University at the time of the research.Now that we have reverse engineered this process, we can fine tune methods of population control with Wolbachia that are already in use. We plan to take advantage of this knowledge to augment currently existing disease vector and pest control methods, and perhaps emulate the technique without Wolbachia or virus proteins in the long-term.
Assistant professor Rupinder Kaur
AbstractIt's hard to think of a more malevolent design than a bacterium that infects a species, bringing along a virus to help it manipulate its hosts reproduction for its own and its viruses benefit, even to the extent of killing developing embryos unless both parents are infected with the same strain of bacterium, so giving infected insects an advantage.
The extent to which prophage proteins interact with eukaryotic macromolecules is largely unknown. In this work, we show that cytoplasmic incompatibility factor A (CifA) and B (CifB) proteins, encoded by prophage WO of the endosymbiont Wolbachia, alter long noncoding RNA (lncRNA) and DNA during Drosophila sperm development to establish a paternal-effect embryonic lethality known as cytoplasmic incompatibility (CI). CifA is a ribonuclease (RNase) that depletes a spermatocyte lncRNA important for the histone-to-protamine transition of spermiogenesis. Both CifA and CifB are deoxyribonucleases (DNases) that elevate DNA damage in late spermiogenesis. lncRNA knockdown enhances CI, and mutagenesis links lncRNA depletion and subsequent sperm chromatin integrity changes to embryonic DNA damage and CI. Hence, prophage proteins interact with eukaryotic macromolecules during gametogenesis to create a symbiosis that is fundamental to insect evolution and vector control.
Rupinder Kaur et al. ,
Prophage proteins alter long noncoding RNA and DNA of developing sperm to induce a paternal-effect lethality.
Science 383, 1111-1117 (2024). DOI:10.1126/science.adk9469
© 2024 The authors. Published by the American Association for the Advancement of Science.
Reprinted with kind permission under licence #5745590955131
But for the most bizarre of reasons, creationists would rather we had that view of their putative designer than understand how this is the work of a mindless natural process not involving supernatural magic.
The Malevolent Designer: Why Nature's God is Not Good
Illustrated by Catherine Webber-Hounslow.
The Unintelligent Designer: Refuting The Intelligent Design Hoax




No comments :
Post a Comment
Obscene, threatening or obnoxious messages, preaching, abuse and spam will be removed, as will anything by known Internet trolls and stalkers, by known sock-puppet accounts and anything not connected with the post,
A claim made without evidence can be dismissed without evidence. Remember: your opinion is not an established fact unless corroborated.