Religion, Creationism, evolution, science and politics from a centre-left atheist humanist. The blog religious frauds tell lies about.
Monday, 23 February 2026
Refuting Creationism - A Small Problem for Science - A Massive Blow For Creationists
Bacteria frozen in ancient underground ice cave found to be resistant against 10 modern antibiotics
As every schoolboy knows, Alexander Fleming discovered the first antibiotic when the fungus Penicillium contaminated a Petri dish in which he had been culturing bacteria. What Fleming had discovered was a naturally occurring antibacterial substance produced by the fungus.
Such compounds are produced by fungi as part of their evolutionary arms race with the bacteria in their environment, and there is a whole range of them, many still awaiting discovery. On the other side of this arms race, bacteria evolve resistance.
It is a struggle that has been going on for hundreds of millions of years, ever since fungi evolved — and perhaps even earlier between ancestral eukaryotes and bacteria. Modern medical use of antibiotics has simply accelerated this ancient contest. We are now facing a major challenge in keeping pace with bacterial evolution, and hospitals in particular have become breeding grounds for resistant strains.
The tendency, therefore, is to assume that antibiotic resistance is a modern, anthropogenic phenomenon. It comes as something of a surprise, then, to learn that a bacterium, Psychrobacter SC65A.3, recovered from 5,000-year-old ice cores in a Romanian cave, has been found to be resistant to ten modern antibiotics.
Frankly, this is difficult to explain other than in terms of earlier evolutionary arms races. The discovery, by a team from the Institute of Biology, Bucharest, Romania, with colleagues from the University of Bucharest and the Universidad de Antofagasta, Chile, is reported in the journal Frontiers in Microbiology.
While this finding presents microbiologists with an intriguing puzzle, it presents creationists with a more acute problem. There simply should not be 5,000-year-old ice preserved in a Romanian cave — let alone viable bacteria within it — if the biblical narrative of a global flood some 4,000 years ago were historically accurate. And if a putative designer deity created bacteria already equipped with resistance to antibiotics that would not be synthesised by humans for millennia, that would imply pre-emptive malevolence.
This leaves modern Intelligent Design advocates with an uncomfortable choice: retreat into literalist theology and abandon scientific reasoning, or confront the implications of the evidence.
Tuesday, 3 February 2026
Unintelligent Design - A Bacterium That Goes Wrong And Self-Destructs

Microbiologists at the University of Southern California (USC) have discovered that one of Earth’s most abundant species, the SAR11 bacterium, has a fundamental — and potentially fatal — ‘design’ flaw. They have just published their findings in Nature Microbiology, and it should make grim reading for any creationists with sufficient courage to read it.
When you have trillions of copies, what does it matter to ‘selfish’ genes if a few billion go wrong and end up destroying the organisms they travel through time in? For an evolved organism, it matters not one tittle or jot to its genes, because they can always produce more copies. So long as there is a sufficiently large population to keep replicating, they will continue to exist and reproduce — and they have no other ultimate function. This is all they evolved to do.
But could we say the same for an organism designed by an omniscient, intelligent designer? What would be intelligent about creating an organism that, under particular but entirely predictable conditions, attempts to reproduce but succeeds only in making repeated copies of its DNA, fails to divide, and enters a runaway cycle of replication until it becomes so disorganised that it can no longer survive and effectively self-destructs?
SAR11 dominates the surface waters of the world’s oceans and accounts for around 40% of marine bacterial cells. As such, it is a vital component at the base of the marine food chain, and is so successful partly because of a process known as genetic streamlining — the evolutionary loss of genes to reduce energy demands in nutrient-poor environments. This alone is not the main problem for creationists to explain, although it does raise the obvious question of why a designer would burden an organism with a genetic load it does not need in the first place.
The real problem is that this streamlining, as an evolved process, comes at a cost. In shedding a load of mostly surplus genes, some essential ones are lost too — including genes that regulate the cell cycle. The result is a failure to divide after genome replication, with the cell instead entering an uncontrolled loop of DNA replication without division.
How on Earth can that be regarded as intelligent design? The organism does exactly what it is ‘designed’ to do under conditions of low nutrient stress, but in doing so falls into an inescapable trap. The consequence is that populations continue to decline even when nutrients later become available again — with potentially serious knock-on effects for other species higher up the food chain.
Monday, 19 January 2026
Refuting Creationism - An Evolutionary Arms Race In Space - No Intelligence Involved
This article is best read on a laptop, desktop, or tablet

In a striking demonstration of the theory of evolution in practice — and something that will have creationists once again insisting on redefining evolution as a theory about one creature turning into an entirely unrelated taxon — an experiment aboard the International Space Station has shown how subtle changes in the environment can dramatically alter an evolutionary trajectory. It also illustrates another major embarrassment for Intelligent Design creationists: evolutionary arms races. Arms races are, of course, utterly incompatible with the idea of an intelligent designer, since running to stand still in a race with yourself is a neat definition of insanity.
Unlike the creationist parody of evolution — carefully engineered to be unprovable because it does not describe what actually happens and what no biologist has ever claimed — the real scientific definition of evolution is simply a change in the frequency of different alleles in a population over time. A definition that creationists know to be irrefutable, hence their persistent attempts to redefine it so that they have something to attack.
The experiment, which had a parallel control on Earth, was designed to observe how bacteriophage viruses that parasitise bacteria and their hosts co-evolved in the microgravity environment of space.
The results have just been published in PLOS Biology. They show that both the T7 phage virus and the E. coli bacteria developed marked genomic differences compared with the Earth-bound populations. The space-station phages gradually accumulated specific mutations that enhanced infectivity or improved their ability to bind receptors on bacterial cells. Meanwhile, the space-station E. coli accumulated mutations that improved resistance to phages and enhanced survival in near-weightless conditions. In other words, what was observed was a genuine evolutionary arms race — and because the environments differed between the space-station populations and the Earth-bound populations, the divergence can be attributed directly to differences in gravity.
The results highlighted another intriguing angle: the mutations that phages and bacteria acquire in space don’t just reveal fundamental evolutionary dynamics, they can also have practical applications for human health on Earth. After 25 days aboard the ISS, both organisms returned with novel mutations not commonly seen under terrestrial gravity, including changes to bacterial surface proteins and corresponding phage adaptations to bind those altered surfaces.
Researchers then engineered phages carrying these space-derived mutations and tested them against bacterial pathogens responsible for urinary tract infections — many of which are now resistant to antibiotics — finding the space-influenced phages were notably effective at killing these otherwise resistant strains. This suggests that the unique selection pressures of microgravity may reveal evolutionary pathways that could be harnessed to design improved therapies for antibiotic-resistant infections back on Earth.
Monday, 2 June 2025
Malevolent Design - The Sneaky Way TB Keeps On Making Us Sick
Study discovers DNA switch that controls TB growth – and could help unlock its antibiotic resistance secrets | University of Surrey
If you're an omniscient, omnipotent, malevolent designer of parasites — such as the bacterium that causes tuberculosis in humans — then you're hardly going to let a little thing like the immune system (which you supposedly also designed to protect them) or even the development of medical science and antibiotics spoil your fun in causing random suffering, are you? Naturally, you'd equip your creation with mechanisms to overcome these obstacles.
Within the framework of Intelligent Design creationism, that's precisely what this recent discovery should look like — at least to those creationists who don't simply ignore the obvious and pretend it isn't there. Scientists from the Universities of Surrey and Oxford have discovered that Mycobacterium tuberculosis uses a reversible process known as ADP-ribosylation to modify its DNA, controlling both replication and gene expression. This allows the bacterium to remain dormant for extended periods and reactivate when environmental threats, such as immune responses or antibiotics, have passed.
This presents a problem for creationists who insist on believing in a benevolent creator deity and simultaneously hold that features such as irreducible complexity and complex specified information are sure signs of intelligent design—claims promoted by Discovery Institute fellows Michael J. Behe and William A. Dembski. Since Mycobacterium tuberculosis displays these very characteristics, so either it was designed specifically to cause suffering, or those characteristics are not the reliable indicators of divine design that Behe and Dembski claim, and their entire argument collapses.
This discovery was recently published open access in The EMBO Journal, and further details are available in the University of Surrey press release:
Wednesday, 27 November 2024
Mallevolent Design - How Salmonella Sneaks Past Our Defences To Make Us Sick
New study shows how salmonella tricks gut defenses to cause infection
There is a simple paradox at the heart of creationism that I have never even seen an attempt to resolve. It all comes from two beliefs: there is only one designer god capable of designing living organisms and that designer god designed us complete with our immune system with which we can attempt to resist attack by pathogens, and that pathogens are not the work of this design, but are the result of 'genetic entropy' and 'devolution' since Adam & Eve let 'sin' into the world. The fact that Michael J. Behe, who invented that excuse, has let slip that ID Creationism is Bible literalism in a lab coat seems to be lost on his followers who still dutifully insist that it is a scientific alternative to evolution and should be taught in school science class (presumably now with the tale of Adam & Eve taught as real history and 'sin' as a real force in science).
The paradox is, did the designer god give Adam & Eve an immune system, or did it design an upgrade when 'sin' allowed pathogens to exist? If the former, it was anticipating and planning for the so-called 'fall'; if the latter, it lacked foresight so is not omniscient.
But however creationists resolve this paradox they still have to explain why the 'intelligently designed' immune system doesn't work very well and why whatever is designing pathogens seems to be able to overcome it.
The nonsense about 'sin', 'the fall', etc., is trivially easy to refute because any improvement in a parasite's ability to parasitise its host can't possibly be regarded as a devolution from some assumed initial perfection because an improvement can't be worse that what it's an improvement on. The whole nonsense of 'devolution' is biological gobbledygook, intelligently designed to appeal to scientifically illiterate simpletons who want to fit the Bible superstition somewhere in the reasoning without bothering too much about the logic or the biology.
So, the paradox boils down to why an intelligent designer would be having an arms race with itself so the parasites it creates can continue to parasitise the victims it creates complete with their immune system it created to stop them. Creationists normally flee in terror at the mere mention of arms races, which is why you'll never see them discussed in the cult literature apart from where pathogens are waved aside as 'caused by sin', blah, blah, blah...
So, it would be refreshing indeed to see a genuine attempt by an intelligent design creationist try to give some rational explanation, and hopefully without giving away the fact that ID creationism is merely Christian fundamentalism in disguise, for the discovery by a new UC Davis Health study that shows how the Salmonella bacteria, a major cause of food poisoning, can invade the gut even when protective bacteria are present.
As an added embarrassment for creationists, Salmonella is closely related to Escherichia coli (E.coli) that they usually cite Michael J. Behe as 'proving' it must have been designed by their god because its flagellum is 'irreducibly complex'.
First a little AI background information about Salmonella, where it came from and what it does to us:
Saturday, 12 October 2024
Malevolent Design - How Chlamydia Is 'Designed' to Cause Maximum Sufferring.

The problem of parasites for creationists is one that, despite the best efforts of apologists like Michael J Behe of the Deception Institute, just won't go away.
Sadly, Behe shot himself in the foot with his original claim to have proven 'intelligent [sic] design in living organisms with his choice of the bacterial flagellum in E. coli, where he persuaded his willing audience that these nasty little pathogens had been intelligently designed - and by unspoken assumption, designed by the locally-popular god.
Now creationists wave his 'proof' of design as evidence for their creator god because only their god is capable of creating living organisms.
But, with characteristic double-think, creationists also argue that their god is omnibenevolent, so something else must have created parasites like E. coli, and, courtesy again of Michael J. Behe, they cite 'Sin' causing 'genetic entropy' and the absurd idea of 'devolution' this supposedly causes, as the cause of parasites and pathogens (but not the bacterial flagellum, obviously!).
The problem with that notion is that they need to do their double-think trick one more time and believe that a trait with improves a pathogens ability to live and reproduce in its host makes it somehow less perfect that one without that trait. So, in the creationist's world, an improvement is a move away from perfection!
But, with a cult that appears to believe learning is a move away from the 'perfection' of pristine ignorance (from whence comes expertise in all aspects of science), that's probably not too difficult a feat of mental gymnastics for a creationist to perform.
Saturday, 31 August 2024
Creationism Refuted - A Marine Relative of Mycobacterium Tuberculosis Shares 80% Of Its Genome

Tell a creationists that humans and chimpanzees have 98% of their genomes in common, and they'll tell you this doesn't prove common origins or 'macro-evolution', but show them evidence that two bacteria have evolved from a common ancestor because they have 80% of their genome in common and they'll tell you this doesn't mean they've evolved because they are both still 'bacteria kind'.
So, why doesn't 98% commonality mean humans and chimpanzees are both still 'ape kind'?
But the evidence that the two bacteria, Mycobacterium tuberculosis, and the newly-discovered M. spongiae is compelling, and gives a clue as to the origins of M. tuberculosis, one of the most deadly pathogenic bacteria, possibly from marine origins.
Saturday, 13 July 2024
Malevolent Designer - How A Respiratory Pathogen Manipulates Our Immune System.
Respiratory bacteria ‘turns off’ immune system to survive - UQ News - The University of Queensland, Australia
Regular readers may recall my recent article explaining how bowel cancer has been cleverly 'designed' to switch off our immune system to prevent the cancer cells from being detected and attacked.
Well, it seems this same technique has been employed by the malevolent designer to make a bacterial pathogen better at making us sick when it infects the lining of the respiratory system of vulnerable people.
Or at least that is what an intellectually honest intelligent [sic] design creationist has to believe (if there is such a thing) because, in rejecting the science that shows how organism's evolve by a mindless, unintelligent natural process without the involvement of supernatural magic, and attributing it all to their putative designer, they are tacitly accepting that it also designs parasites such as these bacterial pathogens.
And we can exclude Michael J. Behe's scientifically nonsensical notions of 'genetic entropy' and 'devolution' [sic] from some assumed created initial perfection - made possible by the religious dogma of 'The Fall', because an ability that conveys an advantage to a pathogen and makes it able to produce more offspring than what went before, can't logically be described as less perfect than something worse, and of course, there is no known mechanism which would cause a detrimental mutation to increase in the species gene pool, other than riding piggyback on a mutation that conveys a greater advantage.
Saturday, 6 July 2024
Refuting Creationism - How A Common Bacterium Became A Dangerous Pathogen - By Evolution, Naturally
Scientists map how deadly bacteria evolved to become epidemic | University of Cambridge
Aficionados of creationism's putative intelligent designer will doubtless be thrilled to discover the imaginative lengths it's gone to to ensure as many people get sick as possible with its cleverly designed bacterium, Pseudomonas aeruginosa, which has gradually been modified over the last 200 years to make it more effective and more resistant to the antibiotics medical science has developed to protect us. There is even a strain specially 'designed' to infect children with cystic fibrosis, just to add to their suffering.
Since these modifications involve genetic mutations which improve the bacterium's ability to love and replicate, we can dismiss the scientific gibberish Michael J Behe invented to explain parasites away as 'devolutionary' and due to 'genetic entropy, since even Michael J Behe would be hard pressed to explain how an advantageous mutation can be less perfect that what went before it. Something better can't be less perfect than something less good, unless you invoke a private definition of 'perfect' that means something which can be bettered.
So, what are these changes in this dangerous pathogen that makes it a problem for people with other health conditions?
Monday, 1 July 2024
Refuting Creationism - More Evidence of Endosybiosis in Progress

Readers my remeber my article about how a team of scientists have discovered a new cell organelle in the process of transforming from a free-living nitrogen-fixing bacterium to becoming an endosymbiont of a marine alga, in much the same way that cyanobacteria became the chloroplasts of plant cells and rickettsia bacteria became the mitochondria of all eukaryote cells.
Now a different team, from the Max Planck Institute for Marine Microbiology, the Alfred Wegener Institute and the University of Vienna, have reported on a similar phenomenon in the form of a bacterium closely related to the Rhizobia that form a symbiotic association with leguminous plants such as peas and bean, which has teamed up with a marine diatom. This symbiotic relationship involves the bacterium living within the single cell of the diatom, unlike the relationship between Rhizobia and legumes in which the bacteria live in special nodes on the roots of the plants, but not inside the plant cells as such.
What is the chemical pathway by which nitrogen-fixing bacteria convert atmospheric nitrogen into ammonia and nitrates? Nitrogen-fixing bacteria play a crucial role in the nitrogen cycle by converting atmospheric nitrogen (N₂) into ammonia (NH₃), which can be further converted into nitrates (NO₃⁻) by other microorganisms. The chemical pathway for nitrogen fixation primarily involves the enzyme nitrogenase, which catalyzes the reduction of atmospheric nitrogen to ammonia. Here is an overview of the pathway:This association in the oceans accounts for the supply of fixed nitrogen in the seas which is then available for plant and ultimately animal life.
- Nitrogen Fixation
Nitrogenase Enzyme Complex:
- Components: The nitrogenase complex consists of two main proteins: the iron (Fe) protein and the molybdenum-iron (MoFe) protein.
- Reaction:
\[ \begin{equation*} \begin{aligned} \text{N}_2 + 8H^+ + 8e^- + 16ATP & \\ \rightarrow 2NH_3 + H_2 + 16ADP + 16Pi & \end{aligned} \end{equation*} \]- Steps:
- Electron Donation: Electrons are donated from ferredoxin or flavodoxin to the Fe protein.
- ATP Hydrolysis: ATP binds to the Fe protein and is hydrolyzed, providing energy for the transfer of electrons.
- Electron Transfer: Electrons are transferred from the Fe protein to the MoFe protein.
- Nitrogen Reduction: The MoFe protein reduces atmospheric nitrogen (N₂) to ammonia (NH₃) in a series of steps involving the binding and reduction of N₂.
Ammonia Assimilation
Once ammonia is produced, it can be assimilated into organic compounds or further processed into other nitrogenous compounds.
Conversion to Nitrates
The conversion of ammonia to nitrates occurs in two steps involving nitrifying bacteria:
Step 1: Ammonia to Nitrite
Bacteria Involved: Ammonia-oxidizing bacteria (AOB), such as Nitrosomonas. \[ \text{NH}_3 + \text{O}_2 \rightarrow \text{NO}_2^- + 3H^+ + 2e^- \] Step 2: Nitrite to Nitrate
Bacteria Involved: Nitrite-oxidizing bacteria (NOB), such as Nitrobacter. \[ \text{NO}_2^- + \frac{1}{2}\text{O}_2 \rightarrow \text{NO}_3^- \]
Summary of PathwayThis pathway is essential for making atmospheric nitrogen available to plants and other organisms in a usable form, thereby sustaining the nitrogen cycle in ecosystems.
- Nitrogen Fixation: Atmospheric nitrogen (N₂) is converted to ammonia (NH₃) by nitrogenase enzyme complex in nitrogen-fixing bacteria.
- Ammonia Assimilation: Ammonia can be incorporated into organic molecules or further processed.
- Nitrification: Ammonia is first oxidized to nitrite (NO₂⁻) by AOB and then nitrite is oxidized to nitrate (NO₃⁻) by NOB.
Of course, any intelligent designer would have given the diatoms the necessary genes to fix nitrogen themselves, just as it would have given legumes the same ability, but evolution, in its haphazard, unplanned and unintelligent way often produces sub-optimal, overly complex solutions to problems simply because that solution was better than what went before; there is no intelligence to think about using processes designed earlier or of the elegance of the solution.
The discovery is the subject of an open access paper in Nature and a press release from Vienna University (Universität Wien):
Long-standing marine mystery solved: How algae get nitrogen to grow
Newly discovered symbiosis between Rhizobia and diatoms could also open new avenues for agriculture
In a new study, scientists from the Max Planck Institute for Marine Microbiology, the Alfred Wegener Institute and the University of Vienna shed light on an unexpected partnership: A marine diatom and a bacterium that can account for a large share of nitrogen fixation in vast regions of the ocean. This symbiosis likely plays a key role for global marine nitrogen fixation and productivity, and thus uptake of carbon dioxide. The newly-discovered bacterial symbiont is closely related to the nitrogen-fixing Rhizobia which live in partnership with many crop plants and may also open up new avenues for engineering nitrogen-fixing plants. The results were published in the current print edition of the renowned journal Nature.
Nitrogen is an essential component of all living organisms. It is also the key element controlling the growth of crops on land, as well as the microscopic oceanic plants that produce half the oxygen on our planet. Atmospheric nitrogen gas is by far the largest pool of nitrogen, but plants cannot transform it into a usable form. Instead, some crop plants like soybeans, peas and alfalfa (collectively known as legumes) have acquired Rhizobial bacterial partners that "fix" atmospheric nitrogen into ammonium, which can be used by plants. This partnership makes legumes one of the most important sources of proteins in food production.
Yet, how marine plants obtain the nitrogen they need to grow has not yet been fully clarified. Scientists from the Max Planck Institute for Marine Microbiology, the Alfred Wegener Institute and the University of Vienna now report that Rhizobia can also form similar partnerships with tiny marine plants called diatoms – a discovery that solves a long-standing marine mystery and which has potentially far-reaching agricultural applications.
An enigmatic marine nitrogen fixer hiding within a diatom
For many years it was assumed that most nitrogen fixation in the oceans was carried out by photosynthetic organisms called cyanobacteria. However, in vast regions of the ocean there are not enough cyanobacteria to account for measured nitrogen fixation. Thus, many scientists hypothesized that non-cyanobacterial microorganisms must be responsible for the "missing" nitrogen fixation.
In 2020, the scientists travelled from Bremen to the tropical North Atlantic to join an expedition involving two German research vessels. They collected hundreds of liters of seawater from the region, in which a large part of global marine nitrogen fixation takes place, hoping to both identify and quantify the importance of the mysterious nitrogen fixer. It took them the next three years to finally puzzle together its genome.For years, we have been finding gene fragments encoding the nitrogen-fixing nitrogenase enzyme, which appeared to belong to one particular non-cyanobacterial nitrogen fixer but, we couldn’t work out precisely who the enigmatic organism was and therefore had no idea whether it was important for nitrogen fixation.
Marcel M. M. Kuypers, lead author
Max Planck Institute for Marine Microbiology, Bremen, Germany
It was a long and painstaking piece of detective work but ultimately, the genome solved many mysteries.
Bernhard Tschitschko, first author
Max Planck Institute for Marine Microbiology, Bremen, Germany Now: Department of Microbiology
University of Innsbruck, Innsbruck, Austria.Based on the nitrogenase gene fragment we had seen in many marine samples before, one would have expected to find this gene in a Vibrio-related organism, but by carefully piecing together the genetic information it turned out that instead, it belonged to a genome closely related to known Rhizobia, which typically live in symbiosis with legume plants.
Daan R. Speth, co-author
Max Planck Institute for Marine Microbiology, Bremen, Germany Now: Centre for Microbiology and Environmental Systems Science
Division of Microbial Ecology
University of Vienna, Vienna, Austria,
Together with its surprisingly small genome, this raised the possibility that the marine Rhizobia might be a symbiont.
The first known symbiosis of this kind
Spurred on by these discoveries, the authors developed a genetic probe which could be used to fluorescently label the Rhizobia.
Indeed, their suspicions about it being a symbiont were quickly confirmed.This allowed us to visualize the Rhizobia directly in their native habitat - the complex environmental samples collected in the Atlantic.
Katharina Kitzinger, co-author
Max Planck Institute for Marine Microbiology, Bremen, Germany
Now: Centre for Microbiology and Environmental Systems Science
Division of Microbial Ecology
University of Vienna, Vienna, Austria.
The scientists named the newly discovered symbiont Candidatus Tectiglobus diatomicola. Having finally worked out the identity of the missing nitrogen fixer, they focused their attention on working out how the bacteria and diatom live in partnership. Using a technology called nanoSIMS, they could show that the Rhizobia exchanges fixed nitrogen with the diatom in return for carbon. And it puts a lot of effort into it:We were finding sets of four Rhizobia, always sitting in the same spot inside the diatoms. It was very exciting as this is the first known symbiosis between a diatom and a non-cyanobacterial nitrogen fixer.
Marcel M. M. Kuypers.
A crucial role in sustaining marine productivityIn order to support the diatom’s growth, the bacterium fixes 100-fold more nitrogen than it needs for itself.
Wiebke Mohr, co-author
Max Planck Institute for Marine Microbiology, Bremen, Germany
Next the team turned back to the oceans to discover how widespread the new symbiosis might be in the environment. It quickly turned out that the newly discovered partnership is found throughout the world’s oceans, especially in regions where cyanobacterial nitrogen fixers are rare. Thus, these tiny organisms are likely major players in total oceanic nitrogen fixation, and therefore play a crucial role in sustaining marine productivity and the global oceanic uptake of carbon dioxide.
A key candidate for agricultural engineering?
Aside from its importance to nitrogen fixation in the oceans, the discovery of the symbiosis hints at other exciting opportunities in the future. Kuypers is particularly excited about what the discovery means from an evolutionary perspective.
The evolutionary adaptations of Ca. T. diatomicola are very similar to the endosymbiotic cyanobacterium UCYN-A, which functions as an early-stage nitrogen-fixing organelle. Therefore, it’s really tempting to speculate that Ca. T. diatomicola and its diatom host might also be in the early stages of becoming a single organism.
Marcel M. M. Kuypers.
Tschitschko agrees that the identity and organelle like nature of the symbiont is particularly intriguing.The scientists will now continue to study the newly discovered symbiosis and see if more like it also exist in the oceans.So far, such organelles have only been shown to originate from the cyanobacteria, but the implications of finding them amongst the Rhizobiales are very exciting, considering that these bacteria are incredibly important for agriculture. The small size and organelle-like nature of the marine Rhizobiales means that it might be a key candidate to engineer nitrogen-fixing plants someday.
Bernhard Tschitschko
Original publication:
Bernhard Tschitschko, Mertcan Esti, Miriam Philippi, Abiel T. Kidane, Sten Littmann, Katharina Kitzinger, Daan R. Speth, Shengjie Li, Alexandra Kraberg, Daniela Tienken, Hannah K. Marchant, Boran Kartal, Jana Milucka, Wiebke Mohr, Marcel M. M. Kuypers (2024): Rhizobia-diatom symbiosis fixes missing nitrogen in the ocean. Nature (2024) DOI: 10.1038/s41586-024-07495-w
Participating institutions:
- Max Planck Institute for Marine Microbiology, Bremen, Germany
- Alfred Wegener Institute - Helmholtz-Centre for Polar and Marine Research, Bremerhaven, Germany
- University of Vienna, Vienna, Austria
Abstract
Nitrogen (N2) fixation in oligotrophic surface waters is the main source of new nitrogen to the ocean1 and has a key role in fuelling the biological carbon pump2. Oceanic N2 fixation has been attributed almost exclusively to cyanobacteria, even though genes encoding nitrogenase, the enzyme that fixes N2 into ammonia, are widespread among marine bacteria and archaea3,4,5. Little is known about these non-cyanobacterial N2 fixers, and direct proof that they can fix nitrogen in the ocean has so far been lacking. Here we report the discovery of a non-cyanobacterial N2-fixing symbiont, ‘Candidatus Tectiglobus diatomicola’, which provides its diatom host with fixed nitrogen in return for photosynthetic carbon. The N2-fixing symbiont belongs to the order Rhizobiales and its association with a unicellular diatom expands the known hosts for this order beyond the well-known N2-fixing rhizobia–legume symbioses on land6. Our results show that the rhizobia–diatom symbioses can contribute as much fixed nitrogen as can cyanobacterial N2 fixers in the tropical North Atlantic, and that they might be responsible for N2 fixation in the vast regions of the ocean in which cyanobacteria are too rare to account for the measured rates.
Main
Nitrogen is an essential component of all living organisms and limits life in the ocean. Atmospheric N2 gas is the largest reservoir of freely accessible nitrogen, but it is biologically available only to microorganisms that carry the nitrogenase metalloenzyme and thus can fix N2 into ammonia7. Even though a wide diversity of marine bacteria and archaea encode nitrogenase, the bulk of nitrogen fixation in the ocean has been attributed to cyanobacteria (ref. 4 and references therein). These phototrophs are capable of both free-living and symbiotic lifestyles, and can directly or indirectly contribute to carbon fixation and export production in the regions where they are abundant, such as oligotrophic coastal waters and margins of subtropical gyres8. Notably, in vast regions of the ocean, such as the centres of subtropical gyres, cyanobacterial N2 fixers are too rare to account for the measured rates of N2 fixation. Instead, a role of non-cyanobacterial N2 fixers has been invoked, on the basis of the abundance of nitrogenase-encoding gene sequences (nifH), most of which belong to uncultured proteobacteria (for example, refs. 3,5,9,10,11). So far, the most frequently detected non-cyanobacterial N2 fixer is the so-called gamma-A, named after its nifH gene phylogeny that clusters within the Gammaproteobacteria12. This enigmatic microorganism has been shown to be distributed in most world oceans, and its potential activity has been inferred from in situ nifH transcription13,14. To date, however, there is no proof that gamma-A fixes N2 in situ, and essentially all aspects of its physiology remain unknown.
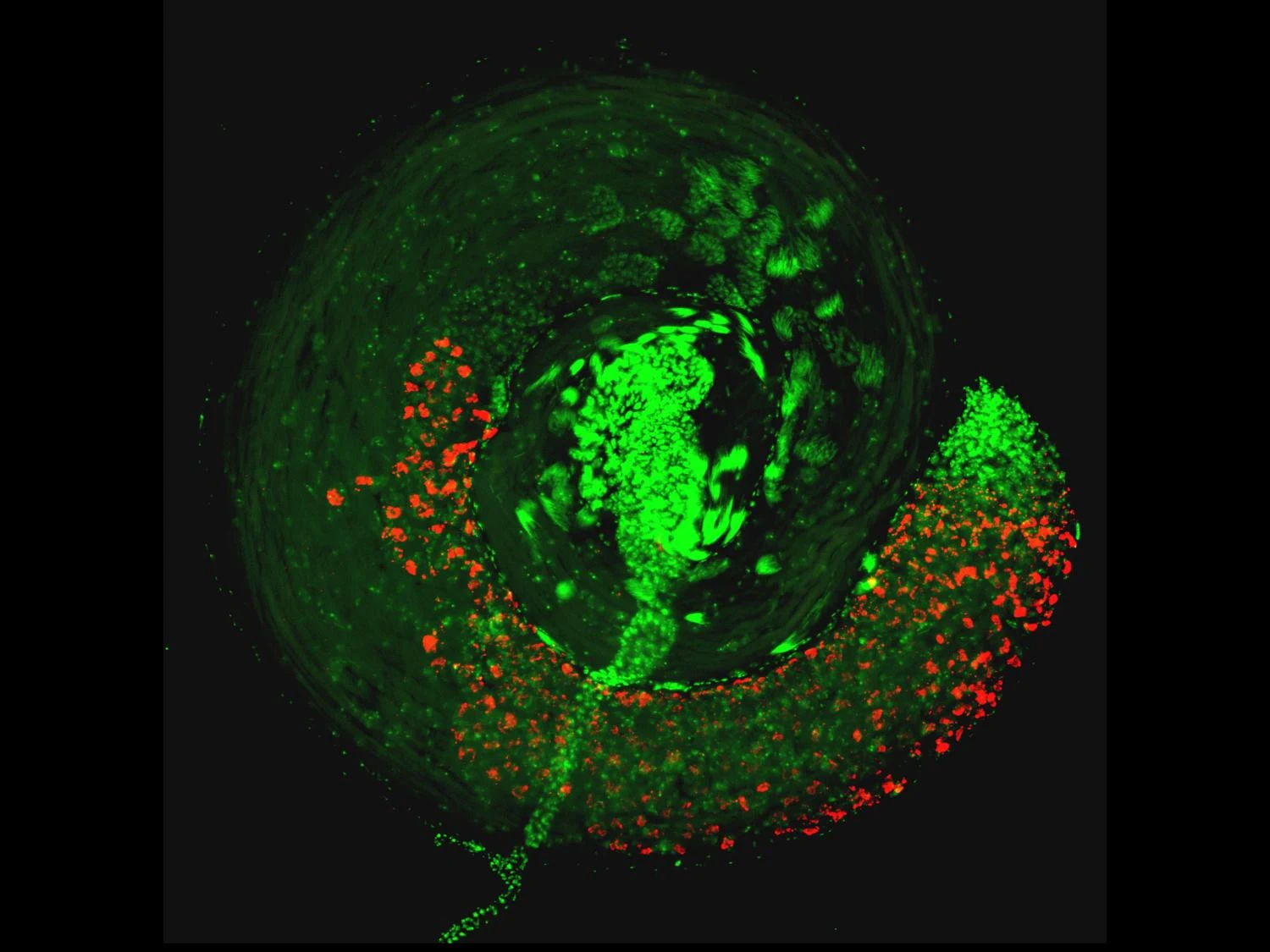
An N2-fixing rhizobial diatom endophyte We investigated the role of non-cyanobacterial N2 fixation in the tropical North Atlantic during an expedition in January–February 2020. This region is responsible for around 20% of oceanic N2 fixation8, and cyanobacteria can only explain approximately half of the rates measured in the region10. We detected high N2 fixation rates of up to 40 nmol N l−1 d−1 in the surface waters (Extended Data Table 1), and the presence of both cyanobacterial and heterotrophic N2 fixers—specifically, gamma-A—was confirmed by metagenomic sequencing (Extended Data Fig. 1a). Gamma-A nifH sequences were retrieved only from the large size fraction (greater than 3 µm) suggesting particle attachment or an association with a host organism (Extended Data Fig. 1a). We recovered a near-complete metagenome-assembled genome (MAG; 1.7 Mb, 37.8% GC, 98% completion with 0% redundancy) containing the gamma-A nifH gene, as well as a complete cluster of rRNA genes (Supplementary Table 1). Although the retrieved nifH sequence clustered within the Gammaproteobacteria as previously reported3,14,15 (Extended Data Fig. 2), both 16S-rRNA-gene-based and whole-genome-based taxonomy16 firmly placed this MAG within the alphaproteobacterial family Hyphomicrobiaceae (Fig. 1a). This family belongs to the order Rhizobiales, which comprises the prominent rhizobial symbionts of nodule-forming terrestrial legumes6,17,18. In addition to nifH, most other genes of the nif regulon are of gammaproteobacterial origin, including nifD and nifK, which encode the catalytic component of the nitrogenase; nifE, nifN and nifB, which encode the iron-molybdenum cofactor assembly proteins; and nifS, which is involved in metallocluster biosynthesis (Extended Data Fig. 2a). Almost all other genes in the gamma-A MAG are of alphaproteobacterial origin (Supplementary Table 1). On the basis of these results, we conclude that the gamma-A N2 fixer is, in fact, an alphaproteobacterium that has acquired its nitrogenase genes through horizontal gene transfer from a gammaproteobacterial donor. Besides gamma-A, several other bacteria, including members of the order Rhizobiales, obtained their nitrogenase genes through horizontal gene transfer from a gammaproteobacterial donor (Extended Data Fig. 2b). Such horizontal gene transfer across classes, resulting in the acquisition of nitrogenase genes, has been reported previously for other N2 fixers19,20.We name the newly discovered species ‘Candidatus Tectiglobus diatomicola’ within a novel genus ‘Candidatus Tectiglobus’ (see Methods for etymology). One other marine MAG from the North Pacific, which we now name ‘Candidatus Tectiglobus profundi’, is affiliated with this novel genus, with 72% average amino acid identity with Ca. T. diatomicola (Supplementary Methods). Compared with their closest relative, a MAG from the Mediterranean Sea, both Ca. Tectiglobus species have a substantially reduced genome size (around 1.7 Mb versus around 5 Mb) and a strongly decreased GC content (around 38% versus around 54%) (Extended Data Fig. 3), which are features typical of endosymbionts21. Notably, a similar reduction in genome size and GC content is observed for the N2-fixing cyanobacterial endosymbiont Candidatus Atelocyanobacterium thalassa, or UCYN-A, which lives in symbiosis with a haptophyte alga22,23. Thus, the genome properties of Ca. T. diatomicola, together with its presence in the large size fraction, strongly indicate a host-associated lifestyle.Fig. 1: Phylogeny and visualization of Candidatus Tectiglobus diatomicola and its diatom host. a, Maximum likelihood phylogenetic tree of concatenated bacterial marker genes from the order Rhizobiales, showing the placement of Ca. T. diatomicola within the Hyphomicrobiaceae family (see Methods). The novel genus Ca. Tectiglobus, comprising Ca. T. diatomicola and its closest relative Ca. T. profundi, is highlighted in pink. Families within the Rhizobiales that contain known N2-fixing legume symbionts and their exemplary host plants are shown. The order Parvibaculales was used as an outgroup. Black dots indicate more than 95% bootstrap support. Scale bar indicates amino acid substitutions per site. Plant icons were designed by Freepik (Neptunia oleracea) or created with BioRender.com. b,c, False coloured scanning electron microscopy (SEM) image (b) and confocal laser scanning microscopy image (c) of a Haslea diatom. Four Ca. T. diatomicola cells (pink, overlay of Hypho1147 and Hypho734 fluorescence in situ hybridization (FISH) probes; Extended Data Table 2) were detected next to the host nucleus (white; stained with DAPI). Scale bars, 5 µm.
a, Maximum likelihood phylogenetic tree of concatenated bacterial marker genes from the order Rhizobiales, showing the placement of Ca. T. diatomicola within the Hyphomicrobiaceae family (see Methods). The novel genus Ca. Tectiglobus, comprising Ca. T. diatomicola and its closest relative Ca. T. profundi, is highlighted in pink. Families within the Rhizobiales that contain known N2-fixing legume symbionts and their exemplary host plants are shown. The order Parvibaculales was used as an outgroup. Black dots indicate more than 95% bootstrap support. Scale bar indicates amino acid substitutions per site. Plant icons were designed by Freepik (Neptunia oleracea) or created with BioRender.com. b,c, False coloured scanning electron microscopy (SEM) image (b) and confocal laser scanning microscopy image (c) of a Haslea diatom. Four Ca. T. diatomicola cells (pink, overlay of Hypho1147 and Hypho734 fluorescence in situ hybridization (FISH) probes; Extended Data Table 2) were detected next to the host nucleus (white; stained with DAPI). Scale bars, 5 µm.
A couple of points here for creationists to ignore or lie about:
- The authors show no doubts that this is an evolutionary process and show no evidence of adopting creationist superstition as a better explanation.
- The endosymbionts have a much reduced genome compared to their free-living relatives, showing that their evolution has involved a loss of genetic information typical of both endosymbionts and endo-parasites, despite the creationist assertion that a loss of genetic information is either fatal or 'devolutionary' [sic]
- This process illustrates the processes involved in the earlier evolution of biodiversity which produced eukaryote cells from associations of prokaryotes, which itself illustrates how 'selfish' genes form co-operative alliances with other 'selfish' genes, despite creationists assertions that selfish genes can only produce selfish individuals.
Wednesday, 26 June 2024
Unintelligent Design - An Over-Complex, Heath-Robinson Solution To A Simple Problem

Imagine you're a designer with all the power you need and all the solutions you've designed earlier at your fingertips and your task is to redesign some marine worms that you designer earlier and put into an arctic environment, perhaps not realising they wouldn't survive if they got frozen.
So, what you have to do is give these marine worms some way to prevent this happening and so mitigating your earlier blunder.
At your disposal is the method you gave to some marine bacteria, and even some fish known as icefish, when you made similar blunders years earlier - you gave them some genes for making antifreeze to stop the contents of their cells freezing and the ensuing ice crystals from destroying them.
Do you give these marine worms the same genes you gave the bacteria?
Not if you're creationism's putative intelligent [sic] designer, you don't. That would be far too simple.
Tuesday, 11 June 2024
Malevolent Designer - How A Dangerous Pathogen Is Designed to Infect Our Lungs

A team at the Biozentrum of the University of Basel have shown how a dangerous pathogen gains access to tissues in the lungs, the better to infect them.
To an intelligent [sic] design creationist, there can only be one explanation for this - intelligent design.
To quickly dispense with the traditional excuse offered for this evidence of malevolence, if you subscribe to the intelligent design nonsense - to blame 'Sin' which was caused by 'The Fall', oblivious of the fact that this exposes the intelligent design argument as fundamentalist religion in disguise. Even given a spurious gloss of sciencey-sounding gibberish by Michael J Behe, with his 'genetic entropy' and 'scientifically nonsensical 'devolution' from some assumed created perfection, makes no sense and fails abysmally to rescue creationism from its obvious religious fundamentalism.
No mutation which conveys an advantage, and so progresses to fixation in the species gene pool, can possibly be regarded as 'devolutionary' or less perfect than what went before it, and of course, postulating some assumed initial created perfection simply underlines the religious fundamentalist and the intellectual contortions needed to try to shoehorn the facts into the superstition inherent in the notion.
So, we are left with malevolent intent as the only conclusion, if we subscribe to the intelligent design notion.
Thursday, 16 May 2024
Malevolent Designer News - Has Creationism's Divine Malevolence Designed An Improved Version of Cholera?
Persistent Strain of Cholera Defends Itself Against Forces of Change, Scientists Find - UT News
One of the mysteries of microbiology and epidemiology, is why a virulent strain of Vibrio cholerae (the bacterium that causes cholera) has remained so stable ever since it emerged in 1961 in Indonesia, causing the seventh global cholera pandemic. this strain, known as 7PET, is now the predominant strain, out-competing the other strains and infecting an estimated 1.3 - 4 million people a year, of which between 21,000 and 143,000 die.
The reason for those wide-ranging estimates is because many of the deaths from cholera are in remoter areas of mostly third-world countries where sanitation is poor, health-care is hard to obtain, and many of the victims are children, so the cause of death is often not known with any certainty.
The traditional response of creationists to anything concerning the evolution of parasites like V. cholerae is to blame 'Sin'. The more sophisticated creationists who have realised that this is a blasphemy because it implies the existence of another creator over which their supposedly omnipotent, omni-benevolent god is powerless, so they simply blame this 'sin' thing for allowing 'genetic entropy' to cause an organism to 'devolve' (it would be a serious blasphemy to call it 'evolution' so Michael J Behe and the Deception Institute had put their heads together and come up with the term 'devolution' instead, which makes it look like the exact opposite of 'evolution'. Stupidly, this devise claims that everything was created perfectly and, when Adam & Eve 'sinned', somehow this opened the door to 'sin', which apparently took their 'omniscient' god by surprise, even though it had given its alleged creations immune systems in anticipation of the effects of 'Sin'.
But of course, we can dismiss that half-baked notion which no serious biomedical scientist would take seriously because there is no mechanism for a deleterious (i.e, 'devolutionarly' trait to accumulate in the species gene pool, and whatever it is about V. cholerae that gives it the edge over other strains, can't rationally be described as 'devolutionary', or a move away from some notional initial perfection, because there can't be anything better than perfect, and yet the 7PET strain of V. cholerae is better at doing the two things it appears to have been designed to do - making more people sick and making more copies of itself than the other strains.
Sunday, 28 April 2024
Uninteligent Design - Bacteria Have A Defence Against The Viruses Designed To Infect Them
Study details a common bacterial defense against viral infection
Creationism's latest hobbyhorse, designed by Michael J Behe to revive the flagging fortunes of his Discovery Institute’s Wedge Strategy and the abysmal failure of his Intelligent [sic] Design ploy to get creationism inserted into the US public school science curricula at US taxpayers' expense, is the silly notion of 'genetic entropy' and 'devolution'.
This unintelligently designed strategy contains the seeds of its own failure at being regarded as a science because it starts off with the assumption that all species were created perfectly by a perfect creator, in compliance with Christian superstition, and so any mutation must be devolutionary away from that perfection. Mutations are called 'genetic entropy', so incorporating the idiotic notion that genetic 'information' is subject to the same law of physics as energy, so the Second Law of Thermodynamic make evolution impossible.
This is supposed to make creationists think Darwin must have been wrong because mutations can't improve fitness, being devolutionary, not evolutionary because of 'genetic entropy'. It ignores the fact that only an improvement in fitness in a given environment can produce more descendants than a deleterious mutation because fitness is defined as the ability to survive and reproduce. How increased fitness can be defined as a reduction in perfection is never explained because Behe 'forgot' to define 'perfection' leaving his marks to assume it means something made by their god.
And this daft notion is now cited by creationists to explain why an intelligent, omniscient and supposedly omnibenevolent god would design parasites. These are now explained as devolved from some initial perfection because of 'genetic entropy' made possible by Adam & Eve's 'sin' - Which is not religious superstition, because it was a historical fact! Got it!
So, we now have an explanation for bacteria, viruses and other parasites, that absolved creationism's god of any responsibility for them while blaming humans for their 'devolution'. However, the problem for this childish nonsense arises when we have parasites on those parasites, such as viruses that infect bacteria - how are bacteria responsible for Adam & Eve's sin and if they aren't why are they being punished for it?
Then it gets even more bizarre when we discover that many bacteria have mechanisms for protecting themselves from these viruses. How can 'devolution' produce an improvement in the organism's ability to survive and reproduce, and in what sense is an improvement a reduction in perfection?
Monday, 11 March 2024
Malevolent Design - How a Bacterium Carries a Virus That Selectively Kills Male Insects And Only Allows Infected Females To Breed
How does a virus hijack insect sperm to control disease vectors and pests? | Penn State University
Wolbachia are a genus of bacteria that form a symbiotic relationship with about 50% of arthropod species, including insects and spiders but they can also manipulate the species for their own ends (in terms of breeding success). They are aided in this by a virus which is incorporated in their genome which has been shown to join forces with Wolbachia to ensure their own reproductive success in the form of females infected with the virus-bearing Wolbachia.
So completely have Wolbachia integrated with insects that one species of fruit fly has the entire Wolbachia genome incorporated into its own genome, making it, biologically, both bacterium and fruit fly.
One way Wolbachia ensure their own survival at the expense of the species of insect they infect is by making the sperm and egg incompatible if the female is not also a carrier of the right species of Wolbachia. And, to be on the safe side, two proteins produced by the virus break the sperm's DNA so any resulting embryo will be defective and will fail to develop. This ensures that only the females carrying the infection can breed, so increasing the Wolbachia and its virus in the gene pool.
The team who discovered this nasty little virus and how it acts selfishly, was co-led by Professor Seth R. Bordenstein, of the One Health Microbiome Center at Pennsylvania State University. They have published their findings in Science and described it in a Penn State News item.
But first, a little background on Wolbachia:
Wednesday, 13 September 2023
Malevolent Designer News - How A Deadly Superbug Is 'Designed' To Make Us Sick

Researchers discover genes behind antibiotic resistance in deadly superbug infections | Doherty Website
Scientists based at the Docherty Institute, Melbourne University, Victoria, Australia have discovered just how devious any designer of the deadly super bug, Staphylococcus aureus, also known as Golden staph, would need to have been to come up with such a clever way to make us sick and die.
Curiously, for people who purport to worship the putative designer of all living things, creationists would have us believe that the designer is just such a mendacious malevolence, rather than have us believe that a natural process such as evolution by natural selection is responsible for parasites and the way they evade our immune systems - also, so creationists claim, designed by the same designer, making it look like a bumbling incompetent amnesiac than a supremely intelligent deity.
First, a brief background on S. aureus and MRSA:
Sunday, 9 July 2023
Creationism in Crisis - Even 'Artificial' Cells With a Minimal Genome Evolve Naturally

University of California at San Diego.
It's turning out to be another terrible week for those few creationists capable, and willing, to read the science.
Here for example is a report on the work of a team led by evolutionary biologist, Professor Jay T. Lennon, of the Department of Biology in the College of Arts and Sciences at Indiana University. It shows a number of things that refute basic creationist dogmas:
- The team use a genetically engineered, stripped down version of the bacterium Mycoplasma mycoides, a parasitic organism that, in common with many parasites had lost genes in the course of its evolution as a dependent parasite in the gut of goats and similar animals, reducing its genome to just 901 genes. So here we have evolution by loss of information (something creationists claim can't happen because their dogma says loss of information is invariably detrimental) as the starting point of this research.
- The team then removed all but the essential genes needed to maintain a functional, reproducing cell, removing a further 45 percent of the genes, reducing the genome to just 493 genes, showing the massive amount of redundancy in a cell's genome - something that no intelligent designer would have designed, showing there was no intelligence in the design of M. mycoides. By comparison, a typical cell can contain 20,000 genes!
- The team then allowed the minimal cell to evolve for 300 days, or 2000 generations (equivalent to 40,000 years of human evolution). They found that even with a minimal genome and so fewer targets for mutation and selection to act on, the minimum cells evolved towards greater fitness, just as the TOE predicts.
- And of course, as with all biological research, there is the complete dependence on the TOE to understand and explain the results, with no hint of doubt in it's explanatory powers or any suggestion that the creationists superstition with its magic and unproven supernatural entity might offer a better explanation of the facts.
Thursday, 13 April 2023
Unintelligent Design - The Complex Lengths to Which Creationism's Idiot Designer Goes To Solve the Problems Its Incompetence Causes
The Complex Lengths to Which Creationism's Idiot Designer Goes To Solve the Problems Its Incompetence Causes
Your baby’s gut is crawling with unknown viruses – University of Copenhagen
If you've bought into the Creation Cult's intelligent [sic] design notion, it's probably best to ignore articles like this that might cause you to doubt the competence of your putative designer, but you're probably used to ignoring evidence that refutes creationism or you wouldn't have fallen for the intelligent [sic] design hoax in the first place.
Nevertheless, it discoveries such as this by Danish Scientists led by Shiraz A. Shah, of Copenhagen University Hospital, Herlev-Gentofte, Gentofte, Denmark, together with colleagues from France and Canada, that highlight the way arms races can lead to ludicrous levels of complexity of which any even half-competent designer would be rightly ashamed.
It comes about because the human gut, especially that of babies, is an ideal, warm, moist, and nutrient-rich environment for micro-organisms, so is teeming with bacteria, protozoa, and viruses, some of which are harmless but some of which parasitize both the microbes and the human cells of the gut.
One incidental benefit that creationists will point to is that the presence of these organisms probably helps train the developing babies’ immune system so it is more effective in later life, so the presence of these organisms can be beneficial.
But hold on! What are they 'training the immune system' for? They are training it to protect us from the very bacteria that the supposedly intelligent designer designed to harm us. At least, since it is supposedly omniscient, it knew what its bacteria and viruses would do when it designed them, so we must assume it designed them for that purpose.
And, if we accept that it designed bacteria to train our immune system, why did it then design the viruses that kill these beneficial bacteria?
But whatever it was thinking of, if 'thinking' is the appropriate term, the result is a baby’s gut teeming with more than 200 different families of virus, many of which were unknown prior to this study. The team's findings are published, open access, in the journal Nature Microbiology.
The research and its significance is explained in a University of Copenhagen news release:
Wednesday, 15 March 2023
Creationism in Crisis - What on Earth was the 'Designer' Thinking?
What on Earth was the 'Designer' Thinking?
The ‘Rapunzel’ virus: an evolutionary oddity

Intra- and inter-ring interactions. A The primary intra-ring interface between two subunits (green and cyan) within a ring. B Schematic quantifying interactions from a single subunit (α) to illustrate the extensive, cooperative network. Numbers and line widths (not lengths) correspond to quantification of the interactions between α and neighboring subunits as calculated by the PDBePISA server (Supplementary Tables 3 and 4). C Surface representation of two subunits reveals a ball and socket geometry between rings. A single subunit (orange) has two loops (Loop1 and Loop2) that fit into sockets (Socket1 - gray, and Socket2 - white) of a subunit in the ring below it (green). D Surface electrostatics of ring interfaces demonstrate an important role for electrostatics in inter-ring interactions.
In addition, Creationists believe one of two things:
- Their god didn't know when he created the mythical founder couple, Adam & Eve, that this would result in this 'sin' thing being created.
- It did know, but created them anyway, presumably not understanding the consequences.
I form the light, and create darkness: I make peace, and create evil: I the Lord do all these things.And they also believe that their god created everything on Earth for his special creation, them of course, or more generally, all humans
Isiah 47:7
Not for the first time, Creationism requires its dupes to believe two or more mutually exclusive things simultaneously.
g a
So, with that in mind, imagine being a Creationist and trying to understand what on Earth your magic god was trying to achieve when it created bacteria, then created the phage viruses to parasitise them, described in the research by a team from the Department of Biochemistry and Molecular Biotechnology, University of Massachusetts Chan Medical School, Worcester MA, USA.
Their research is published open access in the Journal of Biological Chemistry and described in the magazine for the American Society for Biochemistry and Molecular Biology (ASBMB). It describes a phage virus, P74-26, nicknamed the “Rapunzel bacteriophage”, with an extraordinarily long 'tail' which is capable of parasitising even extremophile bacteria that live in hot springs where temperatures can reach 170oC. Neither these bacteria nor the phage virus appear to have any benefit to mankind.
The study was to determine the construction and function of the very long 'tail'.
The discovery was of something that the Creationist idol, Michael J Behe would undoubtedly proclaim as 'irreducibly complex', therefore proof that the locally popular god did it.
To make matters worse for Creationists, the study casually and unintentionally refutes one of the lies that their cult leaders fool them with - that the Theory of Evolution (TOE) is being increasingly rejected by mainstream biologists in favour of their childish, magical and supernatural explanation. The scientists who did the study are in no doubt that the 'tail' is a product of an evolutionary process and that the TOE is a perfect model for explaining and understanding the observed facts.
From the ASBMB magazine:
A recent study in the Journal of Biological Chemistry has revealed the secret behind an evolutionary marvel: a bacteriophage with an extremely long tail. This extraordinary tail is part of a bacteriophage that lives in inhospitable hot springs and preys on some of the toughest bacteria on the planet.
Bacteriophages are a group of viruses that infect and replicate in bacteria and are the most common and diverse things on Earth.
The bacteriophage P74-26 has a tail 10 times longer than most other phage tails and is nearly 1 micrometer long, about the width of some spider’s silk.Angnello, et al.(2023) Journal of Biological ChemistryUnlike many of the viruses that infect humans and animals that contain only one compartment, phages consist of a tail attached to a spiky, prismlike protein shell that contains their DNA.Bacteriophages, or phages for short, are everywhere that bacteria are, including the dirt and water around you and in your own body’s microbial ecosystem as well.
Each phage tail is made up of many small building blocks that come together to form a long tube. Our research finds that these building blocks can change shape, or conformation, as they come together. This shape-changing behavior is important in allowing the building blocks to fit together and form the correct structure of the tail tube.
We used a technique called cryo-electron microscopy, which is a huge microscope that allows us to take thousands of images and short movies at a very high magnification. By taking lots of pictures of the phage’s tail tubes and stacking them together, we were able to figure out exactly how the building blocks fit together.
Bacteriophages are gaining ever-growing interest as an alternative to antibiotics for treating bacterial infections. By studying phage assembly, we can better understand how these viruses interact with bacteria, which could lead to the development of more effective phage-based therapies. … I believe that studying unique, interesting things can lead to findings and applications that we can’t even yet imagine.
Emily Agnello, first author
Department of Biochemistry and Molecular Biotechnology
University of Massachusetts Chan Medical School
Worcester, MA, USA.
Phage tails, like hairstyles, vary in length and style; some are long and bouncy while others are short and stiff. While most phages have short, microscopic tails, the “Rapunzel bacteriophage” P74-26 has a tail 10 times longer than most and is nearly 1 micrometer long, about the width of some spider’s silk. The “Rapunzel” moniker is derived from the fairy tale in which a girl with extremely long hair was locked in a tower by an evil witch.
Brian Kelch, an associate professor of biochemistry and molecular biotechnology at UMass Chan who supervised the work, described P74-26 as having a “monster of a tail.”
Compared with most phages, P74-26 uses half the number of building blocks to form stacking rings that make up the tail.transmission electron micrograph of multiple bacteriophages attached to a bacterial cell wall; the magnification is approximately 200,000.Professor Graham Beards, (Wikimedia Commons)
Phage tails are important for puncturing bacteria, which are coated in a dense, viscous substance. P74-26’s long tail allows it to invade and infect the toughest bacteria. Not only does P74-26 have an extremely long tail, but it is also the most stable phage, allowing it to exist in and infect bacteria that live in hot springs that can reach over 170° F. Researchers have been studying P74-26 to find out why and how it can exist in such extreme environments.
To work with a phage that thrives in such high temperatures, Agnello had to adjust the conditions of her experiments to coax the phage tail to assemble itself in a test tube. Kelch said Agnello created a system with which she could induce rapid tail self-assembly.
The researchers used high-power imaging techniques as well as computer simulations and found that the building blocks of the tail lean on each other to stabilize themselves.I like to think about these phage building blocks as kind of like Legos. The Lego has studs on one side and the holes or sockets on the other. Imagine a Lego where the sockets start off closed. But as you start to build with the Legos, the sockets begin to open up to allow the studs on other Legos to build a larger assembly. This movement is an important way that these phage building blocks self-regulate their assembly.
We think what has happened is that some ancient virus fused its building blocks into one protein. Imagine two small Lego bricks are fused into one large brick with no seams. This long tail is built with larger, sturdier building blocks. We think that could be stabilizing the tail at high temperatures.
Brian A. Kelch, corresponding author
Department of Biochemistry and Molecular Biotechnology
University of Massachusetts Chan Medical School
Worcester, MA, USA
They found P74-26 uses a “ball and socket” mechanism to sturdy itself. In addition, the tail is formed from vertically stacking rings of molecules that make a hollow canal.
Kelch pointed out that, compared with most phages, P74-26 uses half the number of building blocks to form stacking rings that make up the tail.
The researchers now plan to use genetic manipulation to alter the length of the phage tail and see how that changes its behavior.
Phages occupy almost every corner of the globe and are important to a variety of industries like healthcare, environmental conservation and food safety. In fact, long-tailed phages like P74-26 have been used in preliminary clinical trials to treat certain bacterial infections.
AbstractSo, from a Creationist point of view, a massively complex solution to the problem of how to get through the defences of a bacterium that was designed to protect it from viruses in order to kill bacteria that, because they inhabit such extreme conditions, are or complete indifference to humans, being neither harmful nor harmless.
Tail tube assembly is an essential step in the lifecycle of long-tailed bacteriophages. Limited structural and biophysical information has impeded an understanding of assembly and stability of their long, flexible tail tubes. The hyperthermophilic phage P74-26 is particularly intriguing as it has the longest tail of any known virus (nearly 1 μm) and is the most thermostable known phage. Here, we use structures of the P74-26 tail tube along with an in vitro system for studying tube assembly kinetics to propose the first molecular model for the tail tube assembly of long-tailed phages. Our high resolution cryo-EM structure provides insight into how the P74-26 phage assembles through flexible loops that fit into neighboring rings through tight “ball-and-socket”-like interactions. Guided by this structure, and in combination with mutational, light scattering, and molecular dynamics simulations data, we propose a model for the assembly of conserved tube-like structures across phage and other entities possessing Tail Tube-like proteins. We propose that formation of a full ring promotes the adoption of a tube elongation-competent conformation among the flexible loops and their corresponding sockets, which is further stabilized by an adjacent ring. Tail assembly is controlled by the cooperative interaction of dynamic intra- and inter-ring contacts. Given the structural conservation among tail tube proteins and tail-like structures, our model can explain the mechanism of high-fidelity assembly of long, stable tubes.
Agnello, Emily; Pajak, Joshua; Liu, Xingchen; Kelch, Brian A.
Conformational dynamics control assembly of an extremely long bacteriophage tail tube
Journal of Biological Chemistry; (2023). DOI: 10.1016/j.jbc.2023.103021
Copyright: © 2023 The authors.
Published by Elsevier Inc. Open access
Reprinted under a Creative Commons Attribution 4.0 International license (CC BY 4.0)
Creationists also argue that bacteria and viruses were created by 'sin' in some unexplained magical process, so this 'sin' thing is designing parasites to attack the parasites it creates!
It takes some special mental gymnastics to believe these organisms and the relationships between them are the result of an intelligent design process and not the result of a mindless, utilitarian natural process in which neither magic nor magicians was involved.