Mechanism of Plants Obtain Nitrogen by Supplying Iron to Symbiotic Bacteria | Research News - University of Tsukuba
Creationism's 'intelligent' designer is nothing if not inventive. In fact, it's so inventive that it keeps on reinventing things it's already invented and designing new ways to do things it's already designed a way to do. It's almost exactly like it has no way of remembering what it did yesterday and using those designs and inventions today - a bit like a motor-car manufacturer who reinvents the wheel, or the steering mechanism every time it designs a new model.
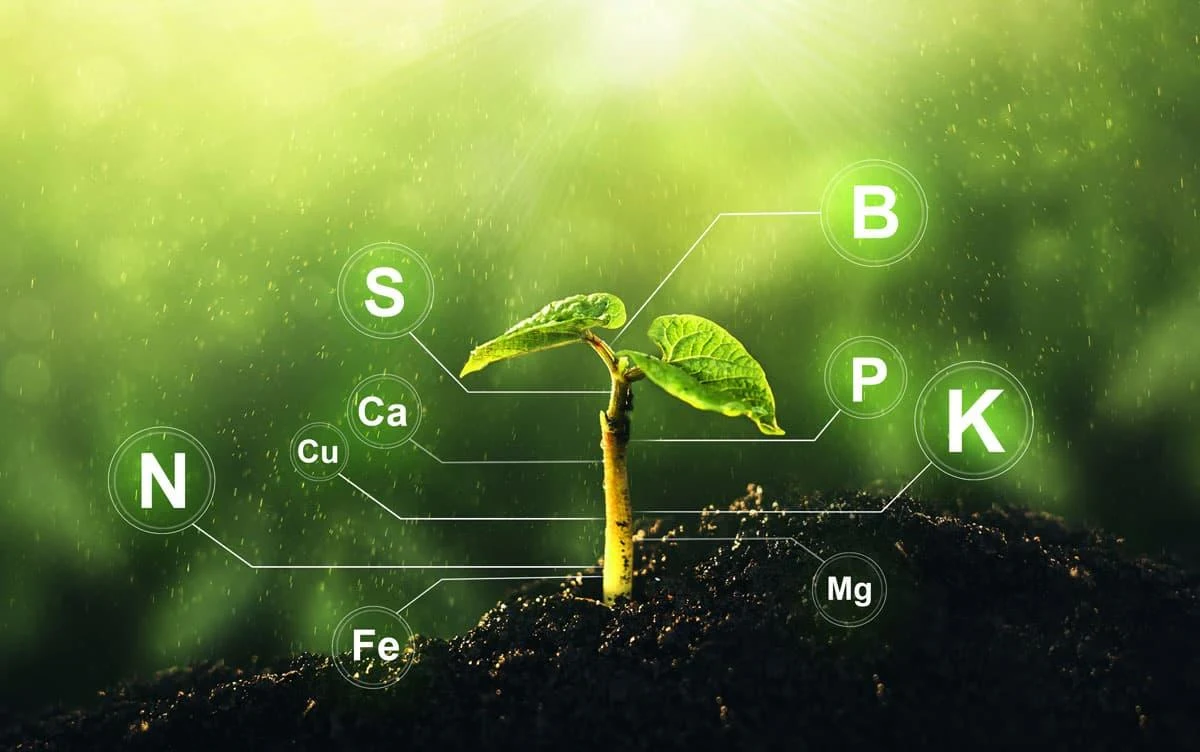
Take, for example, the way most plants obtain the essential nitrates they need to make proteins. Nitrogen is abundant in the atmosphere (about 79%) where it exists as diatomic molecules N2, in which form plants can't assimilate it. Instead they depend on soil bacteria, the 'nitrogen-fixing' bacteria such as Azotobacter and Clostridium which convert N2, into ammonium (NH4+ which forms salts with other minerals in the soil, in which form it can be taken in through the roots of plants. Apparently, it was too simple to give plants the same metabolic pathways that the nitrogen-fixing bacteria have so they could make their own ammonium, so a more complex and less energy-efficient way to get nitrates into plants had to be invented.
A small amount of ammonia (NH3 is also produced by the action of lightening on atmospheric nitrogen and this, together with nitrates produced by industrial pollution is dissolved in rainwater and finds its way into the soil. Other bacteria, fungi and other soil organisms also release nitrates from decaying plant and animal matter in the soil.
How do non-leguminous plants obtain their nitrogen? Non-leguminous plants obtain their nitrogen primarily from the soil in the form of nitrates (NO3-), ammonium ions (NH4+), or organic nitrogen compounds. Unlike leguminous plants, which have a symbiotic relationship with nitrogen-fixing bacteria, non-leguminous plants rely on other mechanisms to acquire nitrogen.But then, not being contented with that method and using it for all land plants, creationism's 'intelligent' designer decided to make it even more needlessly complex for all the members of the legume family (peas, beans, lentils, peanuts, laburnum, broom, mimosa, etc.).Overall, non-leguminous plants employ various strategies to obtain nitrogen from the soil and surrounding environment, enabling them to fulfill their nitrogen requirements for growth and development.
- Uptake from Soil: Non-leguminous plants absorb nitrogen from the soil through their roots. Nitrogen in the soil can exist in various forms, including nitrate (NO3-), ammonium (NH4+), and organic nitrogen compounds. Roots possess specialized transport proteins that facilitate the uptake of these nitrogen compounds into the plant.
- Nitrogen Mineralization: Organic nitrogen compounds present in the soil, such as proteins and amino acids from decaying organic matter, undergo decomposition by soil microorganisms. This process, known as nitrogen mineralization, releases ammonium ions (NH4+) into the soil, which can be taken up by plants.
- Nitrogen Fixation by Free-living Bacteria: While non-leguminous plants do not have symbiotic relationships with nitrogen-fixing bacteria in their roots, certain free-living bacteria in the soil, such as Azotobacter and Clostridium, are capable of nitrogen fixation. These bacteria convert atmospheric nitrogen (N2) into ammonium (NH4+), which can then be utilized by non-leguminous plants.
- Nitrogen Deposition: Non-leguminous plants may also acquire nitrogen through atmospheric deposition. Nitrogen compounds, such as ammonia (NH3) and nitric oxide (NO), can be deposited onto the soil surface through processes like precipitation and atmospheric pollution. These nitrogen compounds can then be taken up by plant roots.
For these, it designed special structures on their roots in which special bacteria called Rhizobia live. These do the work that free-living Azobacter do in the soil:
In the symbiotic relationship between plants of the legume family, how do plants regulate how much nitrate the bacteria supply them with? In symbiotic relationships between legume plants and nitrogen-fixing bacteria (typically Rhizobia), the regulation of nitrate supply is primarily controlled by the plant. This regulation occurs through a process known as nodulation. When a legume plant recognizes the presence of compatible Rhizobia in the soil, it initiates nodulation by releasing specific signaling molecules called flavonoids. These flavonoids attract the Rhizobia to the roots of the plant. In response, the Rhizobia produce another signaling molecule called Nod factor. The Nod factor induces the formation of root nodules, specialized structures where the symbiotic relationship between the plant and bacteria takes place. Inside these nodules, the bacteria are housed in symbiosomes, where they convert atmospheric nitrogen into ammonium through the process of nitrogen fixation. The plant regulates the amount of nitrogen supplied by the bacteria through several mechanisms:There we have a much more complex way to achieve the same thing, and notice that there is already the need for an additional layer of complexity in that one of the enzymes the Rhizobia use needs to be protected from oxygen, almost certainly because it evolved in an anoxic environment before Earth's atmosphere contained oxygen as the waste product of the photosynthetic production of sugar by green plants, showing that they were fixing nitrogen even before the evolution of the cyanobacteria and so are stuck with the problem that the foresight of an omniscient intelligent designer should have foreseen. But, Hey! What's a little bit of additional complexity and additional waste, in pursuit of needless complexity, eh?Overall, the regulation of nitrate supply in the symbiotic relationship between legumes and nitrogen-fixing bacteria is primarily controlled by the plant, which adjusts nodulation and other physiological processes to optimize nitrogen fixation based on its current nitrogen requirements.
- Oxygen Regulation: The plant regulates oxygen flow to the nodules, creating an environment conducive to nitrogen fixation. Oxygen is needed by the bacteria for respiration during nitrogen fixation. By controlling oxygen levels, the plant indirectly influences the activity of the nitrogen-fixing bacteria.
- Nodule Number Regulation: The plant can control the number of nodules formed on its roots. This regulation allows the plant to adjust the overall nitrogen-fixing capacity based on its current nitrogen needs.
- Feedback Inhibition: The plant can also regulate nitrogen fixation by producing compounds that inhibit the activity of the nitrogenase enzyme in the bacteria when sufficient nitrogen is available. This mechanism prevents excess nitrogen fixation when the plant's nitrogen requirements are met.
- Nitrogen Demand Signals: The plant can signal its nitrogen demand to the bacteria through various chemical and physiological mechanisms. This communication ensures that the bacteria provide nitrogen in amounts proportional to the plant's needs.
What is the biochemical pathway which converts molecular nitrogen into ammonia?
The biochemical pathway that converts molecular nitrogen (N2) into ammonia (NH3) is called nitrogen fixation. Nitrogen fixation is a complex process that is primarily carried out by certain bacteria, such as Rhizobia in the root nodules of leguminous plants or free-living nitrogen-fixing bacteria like Azotobacter and cyanobacteria. The key enzyme responsible for catalyzing the conversion of molecular nitrogen into ammonia is nitrogenase. Nitrogenase is a complex enzyme system composed of multiple proteins, including nitrogenase reductase and nitrogenase iron protein, which are involved in the reduction of nitrogen. The overall reaction catalyzed by nitrogenase can be represented as follows: N2 + 8H+ + 8e- + 16ATP → 2NH3 + H2 + 16ADP + 16Pi* In this reaction, molecular nitrogen (N2) is reduced to ammonia (NH3) through the addition of electrons (e-) and protons (H+) in the presence of ATP (adenosine triphosphate) as an energy source. The process also generates hydrogen gas (H2). The reduction of nitrogen by nitrogenase is a highly energy-intensive process, requiring the input of ATP to drive the reaction. Additionally, nitrogenase is highly sensitive to oxygen, so nitrogen-fixing bacteria have evolved mechanisms to protect the enzyme from oxygen, such as residing in anaerobic environments or producing specialized structures like root nodules. Overall, nitrogen fixation plays a crucial role in the global nitrogen cycle by converting atmospheric nitrogen into a form (ammonia) that can be utilized by plants and other organisms, ultimately contributing to the fertility of ecosystems.
*Pi = inorganic phosphate (PO43-)
And now, scientists have discovered even more complexity in this system. Left to their own devices, the Rhizobia just continue making more ammonium than the plant can use, so there needs to be a control mechanism - and it turns out to be broadly similar to the one non-leguminous plants use to regulate the nitrogen-fixing bacteria in the soil.
The discovery was made by a research group consisting of plant biologists from the Universities of Tsukuba, Tokyo and Nagoya, Japan and others, who found that the plants have a process (more complexity) for regulating the supply of iron to the Rhizobia in their nodules using small peptides consisting of about 50 amino acids - the same peptides that no-leguminous plants use to maintain their iron/nitrate balance.
The team have published their findings, open access in the journal Nature Communications and described it in a short Tsukuba University news release:
And, illustrating the additional complexity involved, the team give more technical details in the abstract and introduction to their open access paper in Nature Communications:Researchers led by University of Tsukuba, based on the internal nitrogen status of a leguminous plant, have discovered peptide factors that function in the shoot and root systems to transport iron into the root nodules colonized by nitrogen-fixing bacteria. Moreover, these peptide factors regulate nitrogen homeostasis by maintaining a balance between nitrogen and iron concentrations in plants without rhizobial symbiosis.Tsukuba, Japan—Leguminous plants have a mechanism (rhizobial symbiosis) to efficiently acquire nitrogen, which is an essential macronutrient for growth, through the nitrogen-fixing bacteria rhizobia. Root nodules are organs on plant roots that facilitate the symbiotic relationship. Rhizobia coloniza these nodules and fix nitrogen by converting nitrogen from air into ammonia. Iron is needed for the enzymes that catalyze nitrogen fixation; however, where and how iron is transported to the nodule and used for nitrogen fixation is largely unknown.
In this study, using the legume model plant Lotus japonicus, a transcriptome analysis was performed based on the nitrogen status in the plant body during the rhizobial symbiosis process. IRON MAN (IMA) peptides consisting approximately 50 amino acids were identified, which function systemically (shoot and root systems) to collect iron into the nodules following rhizobial infection.
Furthermore, the function of IMA peptides was analyzed in L. japonicus and Arabidopsis thaliana, a plant devoid of rhizobial symbiosis. In both plant species, the IMA peptides maintained nitrogen homeostasis by obtaining iron in response to increased nitrogen concentrations in the plant body, thereby regulating plant growth.
The research group previously identified a mechanism for regulating rhizobial symbiosis in response to presence of nitrogen in the soil. This study builds on previous studies by clarifying the underlying mechanism of iron acquisition in response to nitrogen, which provides further insight into the mechanisms of plant adaptation to the environment.
These findings are promising for the development of new technologies that contribute to a sustainable society by maximizing the capacity of plants for microbial symbiosis in terms of nutrient use.
AbstractIt is by discovering the needless layers of complexity, compared to what an intelligent designer should come up with if he/she were worthy of the title, that we can tell no such intelligent designer was involved in the production of these structures and systems; instead, the 'designer' was a mindless, utilitarian process in which no intelligence was involved. Once evolution has chanced upon a workable solution that is better than what preceded it, it will be retained, no matter how sub-optimal and no matter that there are better solutions to the same problem that the same process produced in earlier times or even in parallel.
Legumes control root nodule symbiosis (RNS) in response to environmental nitrogen availability. Despite the recent understanding of the molecular basis of external nitrate-mediated control of RNS, it remains mostly elusive how plants regulate physiological processes depending on internal nitrogen status. In addition, iron (Fe) acts as an essential element that enables symbiotic nitrogen fixation; however, the mechanism of Fe accumulation in nodules is poorly understood. Here, we focus on the transcriptome in response to internal nitrogen status during RNS in Lotus japonicus and identify that IRON MAN (IMA) peptide genes are expressed during symbiotic nitrogen fixation. We show that LjIMA1 and LjIMA2 expressed in the shoot and root play systemic and local roles in concentrating internal Fe to the nodule. Furthermore, IMA peptides have conserved roles in regulating nitrogen homeostasis by adjusting nitrogen-Fe balance in L. japonicus and Arabidopsis thaliana. These findings indicate that IMA-mediated Fe provision plays an essential role in regulating nitrogen-related physiological processes.
Introduction
Plants use a variety of strategies to acquire nitrogen, an indispensable macronutrient for all living organisms, thereby adapting to their surroundings1. In Arabidopsis thaliana, the genes encoding C-terminally encoded peptide (CEP) are expressed in the root when plants sense a lack of external nitrogen nutrients2. The produced CEP secreted peptides translocate into the shoot and are recognized by two leucine-rich repeat receptor-like kinases, CEP receptor 1 (CEPR1) and CEPR2, which triggers the production of polypeptides, CEP downstream 1 (CEPD1) and CEPD22,3. The shoot-derived CEPD1/2 activates nitrate transporter 2.1 (NRT2.1) both transcriptionally and post-transcriptionally in the root when nitrate is present in the rhizosphere3,4. This systemic signaling pathway from root to shoot to root contributes to nitrogen acquisition for plants to cope with fluctuating nitrate environments. Meanwhile, legumes can utilize nitrogen in the atmosphere by establishing a symbiotic relationship called root nodule symbiosis (RNS) with nitrogen-fixing rhizobia5. While RNS is largely beneficial for plants, it involves energy-consuming processes. Thus, plants are capable of controlling RNS depending on nitrogen availability6. Recent studies in two model legumes, Lotus japonicus and Medicago truncatula, have improved our understanding of the molecular mechanisms that control RNS in response to external nitrate. The central part of the mechanisms is that nodule inception (NIN)-like protein (NLP) transcription factors (TFs) regulate the positive and negative expression of RNS-related genes7,8,9. Lj/MtNLP1 can additionally modulate RNS by controlling nitrate uptake through Lj/MtNRT2.1 expression10,11. Thus, a greater understanding of the mechanisms in response to external nitrogen nutrients is underway. In addition to such mechanisms responsive to external nitrogen nutrients, it is conceivable that plants regulate physiological processes in response to internal nitrogen status; however, the latter mechanisms remain mostly elusive.
Iron (Fe) is an essential micronutrient to sustain numerous biochemical processes. Hence, plants tightly regulate Fe transport, distribution, and homeostasis according to environmental Fe availability12. In A. thaliana, FER-like iron deficiency-induced transcription factor (FIT) is a key TF in regulating plant responses to Fe deficiency that interacts with basic helix–loop–helix subgroup Ib (bHLH Ib) TFs, including bHLH38/39/100/10113,14,15,16. POPEYE (PYE) belongs to the bHLH IVb subgroup and also regulates plant responses to Fe deficiency17. These bHLH TFs activate the expression of numerous genes required for plants to cope with Fe deficiency. The gene expression of FIT, bHLH Ibs, and PYE are dependent on upstream bHLH IVc TFs, including bHLH34/104/105/11515,16. When plants fulfill their Fe needs, a putative Fe sensor BRUTUS (BTS) acts as an E3 ubiquitin ligase to promote the degradation of bHLH IVc proteins, thereby inhibiting plant responses to Fe deficiency18,19. Iron man (IMA) peptides, non-secreted small proteins, are synthesized both in the shoot and the root under Fe-deficient conditions. The resulting IMA peptides interact with BTS and interfere with its function20,21, which allows FIT, bHLH Ibs, and PYE to activate the expression of the Fe-deficiency-responsive genes. Reciprocal grafting experiments indicate that IMA peptides in the shoot have a systemic function in the Fe deficiency response, whereas those in the root have a local function20.
In RNS, Fe has an essential role in symbiotic nitrogen fixation to function as a cofactor of leghemoglobin, an oxygen-carrying protein required for symbiotic nitrogen fixation, and nitrogenase that catalyzes nitrogen fixation5,22,23. Legumes have a mechanism to accumulate Fe in infected cells of nodules using Fe transporters, including natural resistance-associated macrophage protein 1 and proteins similar to A. thaliana vacuolar iron transporters (VITs) that specifically function in nodules24,25,26. The loss-of-function of these Fe transporters attenuates symbiotic nitrogen fixation, indicating Fe plays an essential function in RNS. In M. truncatula, the nodule-specific cysteine-rich (NCR) 247 peptide confiscates haem groups to promote Fe uptake by rhizobia colonized in nodules27. Although there are few reported cases of TFs involved in Fe signaling in legumes, soybean GmbHLH57 and GmbHLH300, which are orthologous to AtFIT and AtbHLH38/39/100/101, are shown to be involved in the regulation of Fe uptake28. In particular, GmbHLH300 regulates Fe uptake in nodules by controlling the expression of yellow stripe-like 7, encoding a putative Fe transporter29. Despite these progresses in our understanding of the mechanisms by which plants cope with Fe deficiency and those by which plants uptake Fe into nodules, our knowledge is incomplete about the molecular mechanisms of how Fe signaling is activated by rhizobial infection and how Fe is provided to nodules from external and internal sources during RNS.
Here, we show that LjIMA genes are expressed depending on internal nitrogen status during RNS. Functional analyses of LjIMA1 and LjIMA2 peptides reveal a mechanism by which Fe is systemically and root-locally accumulated in nodules. We further show that IMA peptides regulate nitrogen homeostasis by adjusting nitrogen-Fe balance. These findings indicate that IMA-mediated Fe provision plays an essential role in the regulation of nitrogen-related physiological processes.
Ito, M., Tajima, Y., Ogawa-Ohnishi, M. et al.
IMA peptides regulate root nodulation and nitrogen homeostasis by providing iron according to internal nitrogen status.
Nat Commun 15, 733 (2024). https://doi.org/10.1038/s41467-024-44865-4
Copyright: © 2024 The authors.
Published by Springer Nature Ltd. Open access.
Reprinted under a Creative Commons Attribution 4.0 International license (CC BY 4.0)
The Unintelligent Designer: Refuting The Intelligent Design Hoax
ID is not a problem for science; rather science is a problem for ID. This book shows why. It exposes the fallacy of Intelligent Design by showing that, when examined in detail, biological systems are anything but intelligently designed. They show no signs of a plan and are quite ludicrously complex for whatever can be described as a purpose. The Intelligent Design movement relies on almost total ignorance of biological science and seemingly limitless credulity in its target marks. Its only real appeal appears to be to those who find science too difficult or too much trouble to learn yet want their opinions to be regarded as at least as important as those of scientists and experts in their fields.
Available in Hardcover, Paperback or ebook for Kindle
The Malevolent Designer: Why Nature's God is Not Good
This book presents the reader with multiple examples of why, even if we accept Creationism's putative intelligent designer, any such entity can only be regarded as malevolent, designing ever-more ingenious ways to make life difficult for living things, including humans, for no other reason than the sheer pleasure of doing so. This putative creator has also given other creatures much better things like immune systems, eyesight and ability to regenerate limbs that it could have given to all its creation, including humans, but chose not to. This book will leave creationists with the dilemma of explaining why evolution by natural selection is the only plausible explanation for so many nasty little parasites that doesn't leave their creator looking like an ingenious, sadistic, misanthropic, malevolence finding ever more ways to increase pain and suffering in the world, and not the omnibenevolent, maximally good god that Creationists of all Abrahamic religions believe created everything. As with a previous book by this author, "The Unintelligent Designer: Refuting the Intelligent Design Hoax", this book comprehensively refutes any notion of intelligent design by anything resembling a loving, intelligent and maximally good god. Such evil could not exist in a universe created by such a god. Evil exists, therefore a maximally good, all-knowing, all-loving god does not.
Illustrated by Catherine Webber-Hounslow.
Available in Hardcover, Paperback or ebook for Kindle
Illustrated by Catherine Webber-Hounslow.




No comments :
Post a Comment
Obscene, threatening or obnoxious messages, preaching, abuse and spam will be removed, as will anything by known Internet trolls and stalkers, by known sock-puppet accounts and anything not connected with the post,
A claim made without evidence can be dismissed without evidence. Remember: your opinion is not an established fact unless corroborated.