In a paper published today, Ralitsa Madsen of University College London and colleagues show just how devious any designer of one of the more aggressive forms of breast cancer would have to have been, if in fact breast cancer had been designed, as Creationists believe.
The secret to their design is that a mutation makes the cells behave more like stem cells, i.e., cells which have had their epigenetics reset to make them more like the cells from which other, specialist, cells develop during embryonic development. This has been known about for some time but what is new in this research is that there is not one mutation but two or more in the more aggressive cancers and those with a poorer prognosis whereas a single mutation decreases the aggressiveness of the resulting cancer.
The PLOS press release accompanying the open access publication in PLOS Genetics explains:
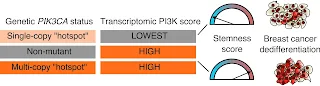
Breast cancers that have an overactive PI3K enzyme, involved in cell growth and division, tend to be more aggressive and to spread and divide more like stem cells. But a new study by Ralitsa Madsen of University College London and colleagues publishing November 11th in the journal PLOS Genetics uncovers a surprising relationship between PI3K activity and mutations in the PIK3CA gene that codes for the enzyme. Breast cancer tumors with one mutant copy of the PIK3CA gene tend to have lower PI3K activity. In comparison, patients with two or more copies often had higher PI3Kα activity, resulting in more aggressive tumors and a poorer prognosis for patients with certain types of breast cancer.In the abstract and summary of their paper, the authors say:
Breast cancer stratification by PIK3CA mutant dose reveals a counterintuitive relationship with functional indices of PI3K pathway activity and tumor dedifferentiation.Experiments in the lab previously showed that two but not one mutant PIK3CA gene can promote a persistent stem cell state—a quality called "stemness". But until now, there was no evidence from human patients to support this idea. In the new study, researchers investigate the relationship among PI3K mutations, PI3K activity and stemness in breast cancer. They used publicly available data from nearly 3,000 breast cancer tumors and applied computational methods to infer PI3K activity and stemness. They discovered that aggressive tumors had more PI3K activity and a higher degree of stemness. However, they were surprised to find that cancer cells with only one mutant copy of PIK3CA had lower levels of stemness and are potentially less aggressive.
Ralitsa Madsen, Lead author
University College London
The new study supports the idea that overactive PI3K enzymes are linked to more aggressive breast cancers. Additionally, the researchers warn that the number of copies of mutant PIK3CA mutations in a tumor may affect how it responds to cancer therapies. They conclude that this information, along with data on PI3K activity, should be considered when choosing patients to participate in clinical trials of new drugs.
AbstractThere are a couple of ways to interpret this from an intelligent [sic] design perspective:
A PI3Ka-selective inhibitor has recently been approved for use in breast tumors harboring the gene encoding PI3Ka. Preclinical studies have suggested that the PI3K/AKT/mTOR signaling pathway influences stemness, a dedifferentiation-related cellular phenotype associated with aggressive cancer. However, to date, no direct evidence for such a correlation has been demonstrated in human tumors. In two independent human breast cancer cohorts, encompassing nearly 3,000 tumor samples, transcriptional footprint-based analysis uncovered a positive linear association between transcriptionally-inferred PI3K/AKT/mTOR signaling scores and stemness scores. Unexpectedly, stratification of tumors according to PIK3CA genotype revealed a "biphasic" relationship of mutant PIK3CA allele dosage with these scores. Relative to tumor samples without PIK3CA mutations, the presence of a single copy of a hotspot PIK3CA variant was associated with lower P13K/AKT/mTOR signaling and stemness scores, whereas the presence of multiple copies of PIK3CA hotspot mutations correlated with higher P13K/AKT/mTOR signaling and stemness scores. This observation was recapitulated in a human cell model of heterozygous and homozygous PIK3CAH1047R expression. Collectively, our analysis (1) provides evidence for a signaling strength-dependent P13K-stemness relationship in human breast cancer; (2) supports evaluation of the potential benefit of patient stratification based on a combination of conventional P13K pathway genetic information with transcriptomic indices of P13K signaling activation.
Author summary
Breast cancers often have increased activity of the so-called PI3Kα. enzyme and the pathway it activates, usually attributed to genetic alterations in the PIK3CA gene, encoding a critical PI3Kα component. Recent cell studies have shown that effects of a PIK3CA mutation depend on how many copies are present. For example, two copies of a strong mutation, but not one, fix cells in a state of "stemness", a property associated with tumor aggressiveness and therapy failure. To determine relationships among PI3K genetic variation, PI3K activity and stemness in breast cancers we used data from independent patient cohorts encompassing nearly 3,000 tumors. Using PI3K signaling or stemness scores derived from gene expression data, we found a strong, positive association between the scores: aggressive tumors show the highest scores. In contrast, the relationship of these scores with PIK3CA mutation status was unexpected - cancers with one PIK3CA mutant copy showed a decrease in both scores, while they increased in cancers with additional copies. This was confirmed in cellular models. This suggests that using information about a PIK3CA mutation alone to define patient groups for trials may miss important effects of mutation number. We suggest that groupings may be improved by combining PIK3CA mutational information with functional indices of PI3K pathway activation.
Madsen RR, Erickson EC, Rueda OM, Robin X, Caldas C, Toker A, et al. (2021)
Positive correlation between transcriptomic stemness and PI3K/AKT/mTOR signaling scores in breast cancer, and a counterintuitive relationship with PIK3CA genotype.
PLoS Genet 17(11): e1009876. https://doi.org/10.1371/journal.pgen.1009876
Copyright: © 2021 The authors. Published by PLoS
Open access
Reprinted under a Creative Commons Attribution 4.0 International license (CC BY 4.0)
- These mutations arise because the entire genome is replicated in every cell, regardless of which genes the specialist cell is going to use. The unwanted genes are then turned off by a complicated system of epigenetics. This ludicrously complex process is subject to error, and when it does go wrong, a cancer can be the result. This should have been forseen by any omniscient designer god.
- The designer god did forsee these problems for its creation and created them anyway, knowing they would cause breast cancers. And when it found that only one mutation didn't work, it came up with two or more to achieve the desired outcome. One 'mistake' is simple carelessness, but two, in full knowledge that one wasn't enough, looks like bloody-minded malevolence.


No comments :
Post a Comment
Obscene, threatening or obnoxious messages, preaching, abuse and spam will be removed, as will anything by known Internet trolls and stalkers, by known sock-puppet accounts and anything not connected with the post,
A claim made without evidence can be dismissed without evidence. Remember: your opinion is not an established fact unless corroborated.