Small Animals Acquire Genes from Bacteria that Can Produce Antibiotics | Marine Biological Laboratory

A. Because creationism isn't a problem for science, but science is a problem for creationism.
It's a basic article of faith for creationists, promulgated by frauds who misrepresent information theory and conflate it with the laws of thermodynamics that an organism cannot acquire new genetic information without the intervention of a supernatural deity (who just happens to be the locally popular god as revealed in the locally popular books deemed to be holy by the locally popular church, mosque, synagogue, temple or gurdwara} because that would violate some assumed law akin to the law of conservation of energy - which has nothing to do with information or genetics
It is of course nonsense, as the evidence of gene and whole genome doubling, and horizontal gene transfer shows. Organisms can acquire new genetic information by simple chemistry and physics; and that new information is often available to be exapted for novel purposes.
Information about bdelloid rotifers, please. Bdelloid rotifers are a fascinating group of microscopic, aquatic animals belonging to the phylum Rotifera. They are known for their unique biological and reproductive characteristics. Here are some key points about bdelloid rotifers:But the example these scientists have provided is of the latter - horizontal gene transfer, this time from bacteria to bdelloid rotifers.
Morphology and Habitat
Reproduction and Genetics
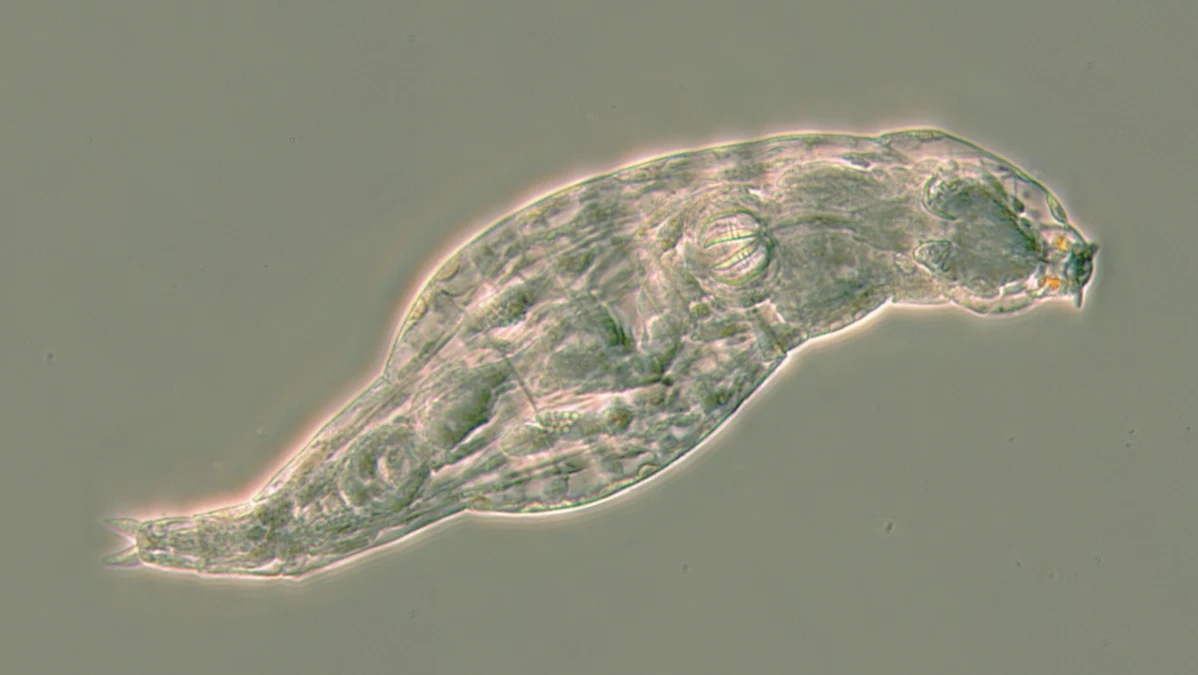
- Size and Structure: Bdelloid rotifers are typically about 150-700 micrometers in length. They have a bilaterally symmetrical, elongated body covered with a cuticle. They possess a characteristic corona, a ciliated structure used for locomotion and feeding.
- Habitat: They are found in a wide range of moist environments, including freshwater, marine habitats, soil, and even mosses and lichens. They can survive in extreme conditions, including desiccation and freezing.
Desiccation Resistance
- Asexual Reproduction: Bdelloid rotifers are known for their asexual reproduction through parthenogenesis, where females produce offspring from unfertilized eggs. No males have been observed in this group.
- Genetic Diversity: Despite their asexual reproduction, bdelloid rotifers exhibit high genetic diversity. This is believed to be partly due to horizontal gene transfer, where they incorporate genetic material from other organisms, and partly due to their ability to survive desiccation, which may allow for DNA repair and recombination.
Ecological Role
- Anhydrobiosis: Bdelloid rotifers can enter a state of anhydrobiosis, where they lose almost all their water content and become dormant. In this state, they can withstand extreme conditions such as radiation, desiccation, and freezing.
- Recovery: When favorable conditions return, bdelloid rotifers can rehydrate and return to their active state, resuming normal biological functions.
Evolutionary Significance
- Decomposers: Bdelloid rotifers play a crucial role in the decomposition of organic matter, contributing to nutrient cycling in aquatic ecosystems.
- Prey and Predator: They serve as a food source for various micro-predators and also feed on microorganisms such as bacteria, algae, and protozoa.
Research Applications
- Ancient Lineage: Bdelloid rotifers are an ancient lineage, with fossil records indicating their presence for hundreds of millions of years.
- Evolution without Sex: They challenge traditional evolutionary theories that emphasize the importance of sexual reproduction for genetic diversity and long-term survival.
Bdelloid rotifers continue to intrigue scientists with their resilience, adaptability, and evolutionary success despite their asexual mode of reproduction. Their study provides insights into alternative strategies for survival and adaptation in the natural world.
- Genomics: Bdelloid rotifers are studied for their unique genomic features, including their ability to repair and maintain their genome under extreme conditions.
- Biotechnology: Their desiccation resistance mechanisms are of interest for developing preservation techniques for biological materials and other applications.
Are bdelloid rotifers multicellular organisms?
Yes, bdelloid rotifers are multicellular organisms. They are small, but they have a complex body structure made up of multiple cells that are organized into tissues and organs. These cells work together to perform various functions necessary for the rotifer's survival and reproduction.
Key Features of Bdelloid Rotifers as Multicellular Organisms:
The multicellular nature of bdelloid rotifers allows them to exhibit behaviors, physiological processes, and ecological roles that are more complex than those of unicellular organisms.
- Cellular Organization: Bdelloid rotifers have a defined body plan with specialized cells that form tissues and organs.
- Corona: The ciliated structure used for locomotion and feeding.
- Mastax: A muscular pharynx with jaws (trophi) for grinding food.
- Digestive System: Includes a stomach and intestine for processing food.
- Nervous System: Consists of a brain (cerebral ganglion) and nerves.
- Excretory System: Involves protonephridia with flame cells for waste removal.
- Reproductive System: Consists of ovaries that produce eggs for parthenogenesis.
- Tissue Differentiation: Bdelloid rotifers exhibit differentiation of cells into specific tissues, such as epithelial tissue lining the body, muscle tissue for movement, and neural tissue for sensory and control functions.
- Organ Systems: Despite their small size, bdelloid rotifers possess distinct organ systems that coordinate to maintain homeostasis, respond to environmental changes, and ensure reproduction.
- Development: Bdelloid rotifers develop from a fertilized egg into a multicellular adult through a process of cell division and differentiation, showcasing their complexity as multicellular organisms.
Rotifers are well known for their ability to incorporate genes from other organisms into their genome, possibly aided in this by their ability to desiccate in dry conditions, then to repair and reassemble their genomes when they rehydrate.
And why the research team have shown is that they have acquires hundreds of anti-fungal genes from bacteria that bacteria originally evolved in their perpetual arms race with fungi, which bdelloid rotifers have the ability to turn on and off according to need.
The team's discovery is the subject of an open access paper in Nature Communications and a news release from The University of Chicago, Marine Biological Laboratory:
Small Animals Acquire Genes from Bacteria that Can Produce Antibiotics
WOODS HOLE, Mass. — A group of small, freshwater animals protect themselves from infections using antibiotic recipes “stolen” from bacteria, according to new research by a team from the University of Oxford, the University of Stirling and the Marine Biological Laboratory (MBL), Woods Hole.
The tiny creatures are called bdelloid rotifers, which means ‘crawling wheel-animals.’ They have a head, mouth, gut, muscles and nerves like other animals, though they are about a human hair's width in size. When these rotifers are exposed to fungal infection, the study found, they switch on hundreds of genes that they acquired from bacteria and other microbes. Some of these genes produce resistance weapons, such as antibiotics and other antimicrobial agents, in the rotifers. The team reports its findings this week in Nature Communications.
When we translated the DNA code to see what the stolen genes were doing, we had a surprise. The main genes were instructions for chemicals that we didn’t think animals could make — they looked like recipes for antibiotics.
Prior research found that rotifers have been picking up DNA from their surroundings for millions of years, but the new study is the first to discover them using these genes against diseases. No other animals are known to “steal” genes from microbes on such a large scale.
These complex genes – some of which aren’t found in any other animals – were acquired from bacteria but have undergone evolution in rotifers. This raises the potential that rotifers are producing novel antimicrobials that may be less toxic to animals, including humans, than those we develop from bacteria and fungi.
David B. Mark Welch, co-author
Josephine Bay Paul Center for Comparative Molecular Biology and Evolution
Marine Biological Laboratory
Woods Hole, MA, USA.
Recipes for self-defense Like other animals, rotifers need strategies to fight off infections and avoid ending up like this diseased individual, which has been taken over and killed by a fungus.Credit: C. G. Wilson
Like other animals, rotifers need strategies to fight off infections and avoid ending up like this diseased individual, which has been taken over and killed by a fungus.Credit: C. G. Wilson
Antibiotics are essential to modern healthcare, but most of them were not invented by scientists. Instead, they are produced naturally by fungi and bacteria in the wild, and humans can make artificial versions to use as medicine.
The new study suggests that rotifers might be doing something similar.
These strange little animals have copied the DNA that tells microbes how to make antibiotics. We watched them using one of these genes against a disease caused by a fungus, and the animals that survived the infection were producing 10 times more of the chemical recipe than the ones that died, indicating that it helps to suppress the disease.
Christopher G. Wilson.
The scientists think that rotifers could give important clues in the hunt for drugs to treat human infections caused by bacteria or fungi.
Antibiotics are becoming less effective because the disease-causing microbes have evolved to become resistant and no longer respond to treatment. The World Health Organization recently sounded the alarm, warning in a June report of the “pressing need” to develop new antibiotics to counter the threat of resistance.
The recipes the rotifers are using look different from known genes in microbes. They’re just as long and complicated, but parts of the DNA code have changed. We think the recipe has been altered by a process of evolution to make new and different chemicals in the rotifers. That’s exciting because it might suggest ideas for future medicines.
Reuben W. Nowell, first author
Biological and Environmental Sciences
University of Stirling, Stirling, Falkirk, UK.
The genes the rotifers acquired from bacteria encode an unusual class of enzymes that assemble amino acids into small molecules called non-ribosomal peptides.
The next phase of this research should involve identification of multiple non-ribosomally synthesized peptides produced by bdelloid rotifers, and establishment of the conditions upon which the synthesis of these compounds can be induced.
Irina R. Arkhipova, co-author
Josephine Bay Paul Center for Comparative Molecular Biology and Evolution
Marine Biological Laboratory
Woods Hole, MA, USA.
One problem with developing new drugs is that many antibiotic chemicals made by bacteria and fungi are poisonous or have side-effects in animals. Only a few can be turned into treatments that clear harmful microbes from the human body.
If rotifers are already making similar chemicals in their own cells, they could lead the way to drugs that are safer to use in other animals, including people.
Why do rotifers acquire so many foreign genes?
A big question is why rotifers are the only animals that borrow these useful genes from microbes at such high rates.
According to one theory, animals that copy themselves like this can become so similar that it starts to be unhealthy. “If one catches a disease, so will the rest,” explained Barraclough. Because bdelloid rotifers don’t have sex, which allows the parental genes to recombine in beneficial ways, the rotifer mother’s genome is directly transferred to her offspring without introducing any new variation.We think it might be linked with another strange fact about these rotifers. Unlike other animals, we never see male rotifers. Rotifer mothers lay eggs that hatch into genetic copies of themselves, without needing sex or fertilization.
If rotifers don’t find a way to change their genes, they could go extinct. This might help explain why these rotifers have borrowed so many genes from other places, especially anything that helps them cope with infections.
Timothy G. Barraclough, co-author
Department of Biology
University of Oxford, Oxford, UK.Nowell thinks there is much more to learn from rotifers and their stolen DNA.These rotifers are crawling around next to a single human hair that has been added to their dish to show the scale. They have laid several small oval-shaped eggs, which will hatch into genetic clones of their mother.Credit: C.G. Wilson, 2024
The rotifers were using hundreds of genes that aren’t seen in other animals. The antibiotic recipes are exciting, and some other genes even look like they’ve been taken from plants. The findings are part of a growing story about how and why genes get moved between different kinds of life.
Reuben W. Nowell.Video Credit: Emily Greenhalgh, MBL
AbstractAlthough rotifers are a problem for evolutionary biology in that they are parthenogenic, so don't have the recombination which gives genetic diversity and 'evolvability' to sexually-reproducing organisms, they are a bigger problem for creationists because they refute a basic dogma of creationism by acquiring new genetic information without magic. By acquiring new genetic information from other organisms in their environment, and by reconstructing their genomes following dessication, they manage to maintain sufficient genetic diversity to avoid the pitfalls of cloning, which limits the organism's ability to evolve in response to evolving predators.
Coevolutionary antagonism generates relentless selection that can favour genetic exchange, including transfer of antibiotic synthesis and resistance genes among bacteria, and sexual recombination of disease resistance alleles in eukaryotes. We report an unusual link between biological conflict and DNA transfer in bdelloid rotifers, microscopic animals whose genomes show elevated levels of horizontal gene transfer from non-metazoan taxa. When rotifers were challenged with a fungal pathogen, horizontally acquired genes were over twice as likely to be upregulated as other genes — a stronger enrichment than observed for abiotic stressors. Among hundreds of upregulated genes, the most markedly overrepresented were clusters resembling bacterial polyketide and nonribosomal peptide synthetases that produce antibiotics. Upregulation of these clusters in a pathogen-resistant rotifer species was nearly ten times stronger than in a susceptible species. By acquiring, domesticating, and expressing non-metazoan biosynthetic pathways, bdelloids may have evolved to resist natural enemies using antimicrobial mechanisms absent from other animals.
Introduction
Antagonistic interactions among species are strong, ubiquitous and relentless sources of selection in natural populations1,2,3. Examples include arms-races between pathogen virulence factors and host immune systems, and coevolution between antimicrobial compounds or toxins and pathways to resist them. These dynamics are central to various global challenges, including emerging infectious diseases, management of crop pathogens, and antimicrobial resistance. According to theory, selection arising from antagonistic coevolution can favour adaptations to shuffle existing combinations of genes or to acquire genes bringing new functions. These processes help explain the especially rapid and intense adaptive evolution4 seen at loci encoding the molecular mediators of conflict5.
Different domains of life typically address the challenge of ongoing genetic mixing in distinct ways. In bacteria and archaea, horizontal gene transfer (HGT) occurs by various mechanisms6,7 and is well known as a route for the spread of antimicrobial resistance8, as well as genes encoding the production of antibiotics and other molecular weapons9. For example, nonribosomal peptide synthetases (NRPS) and polyketide synthetases (PKS) are large, multimodular enzymes that catalyse assembly of a vast array of natural products, including toxins, immunosuppressants and antimicrobial compounds10. These can be encoded as biosynthetic gene clusters on plasmids11 as well as chromosomes, and their mobility and ‘assembly line’ structure facilitate diversification to produce novel secondary metabolites via recombination of modules within and between genomes10. The natural reservoir of mobile genetic diversity for antibiotic synthesis and resistance is thought to reflect a history of coevolution between the producers and targets of antimicrobial compounds12.
In contrast, among eukaryotes, the most important mechanism of genetic exchange is meiotic sex, by which whole genomes are shuffled every generation though recombination, segregation and outcrossing. Although meiotic shuffling has different effects to HGT13, sex too has been linked to biotic conflict because it can speed up host adaptation against pathogens by generating new combinations of resistance alleles14,15. When coevolving pathogens are common and virulent16, the theoretical benefits of genetic exchange are so substantial that they may outweigh the inherent costs17 of sexual reproduction compared with parthenogenesis18,19,20. Antagonistic coevolution may therefore help explain why obligately asexual plant and animal lineages are typically rare and short-lived despite major advantages21,22. This so-called ‘Red Queen Hypothesis’3 (RQH) draws support from associations between recombination and immunity, from host-pathogen dynamics in mixed sexual and asexual populations23,24, and from the susceptibility of asexually propagated lineages to pathogens25.
Here, we investigate the links between genetic transfer and biotic conflict in a group of animals that challenge typical distinctions between domains described above. Bdelloid rotifers are a class of microscopic, filter-feeding invertebrates that live in freshwater and limnoterrestrial habitats worldwide. Reproduction is only known by parthenogenetic eggs, produced by a modified, nonreductional meiosis26. Neither males nor sperm have been reported despite centuries of microscopic observation and the description of hundreds of species27, leading to the hypothesis that the class Bdelloidea has diversified for tens of millions of years in the absence of sexual reproduction28,29. Genetic evidence to either confirm or refute obligate asexuality in bdelloids has proved complicated30, and seemingly definitive evidence both for and against this hypothesis31,32,33,34,35 has been overturned or reinterpreted by later work36,37,38,39,40,41,42. In contrast, repeated studies have demonstrated that bdelloid genomes encode extraordinarily high proportions of genes acquired horizontally from non-metazoan taxa30. Approximately 10% of genes appear to have been captured from bacteria, fungi, plants and other sources, rather than sharing recent common ancestry with metazoan orthologs33,43,44. This estimate is an order of magnitude greater than for other animals45, holds for all bdelloid genomes so far examined, and is consistent across various methods for detecting HGT37,46,47,48.
Comparisons among bdelloid species indicate that most HGT events are ancient, with ongoing acquisition rates estimated to be on the order of one gene per 100,000 years49. At these rates, the phenomenon would be too slow to equate with the sexual shuffling seen in typical eukaryotes, or the rapid dynamics of bacterial accessory genomes. Nevertheless, HGT has been hypothesised to introduce novel biochemical functions that help bdelloids adapt to environmental challenges, as it does in bacteria. Acquired genes are expressed and incorporated into metabolic pathways44,46, some of which are not shared by other metazoans50. Putative functions identified to date include desiccation tolerance, nutrient exploitation and repair of DNA damage44,46,51,52. However, the deep associations between genetic transfer and coevolution raise the hypothesis that HGT may help bdelloids deal with biotic antagonism; for instance by acquiring genes with pathogen resistance functions. Isolated examples of horizontally acquired genes contributing to immunity are known from invertebrates53,54,55, but the massive scale of HGT in bdelloids and the prevalence of asexual reproduction might especially favour the co-option of unusual pathways to resist microbial enemies, with parallels to the role of HGT in bacterial conflict56,57. If so, this could compensate in part for the challenge that an asexual lineage theoretically faces from pathogens.
We investigated this hypothesis by testing whether horizontally acquired genes show a potential enrichment in the response of bdelloid rotifers to infection. Like all animals, bdelloid rotifers are exploited by a range of natural enemies, including over 60 species of virulent fungal and oomycete pathogens58. These can exterminate cultured populations in a few weeks59,60, and significantly depress the abundance of hosts in natural habitats61. However, almost nothing is known about variation in susceptibility among rotifers, how this compares with observations in other invertebrate pathosystems62,63,64,65,66,67, or how the underlying mechanisms evolve and remain effective if sex is rare or absent.
We used RNA-seq to identify genes that are differentially expressed when bdelloid rotifers are attacked by a natural fungal pathogen in the genus Rotiferophthora68 (Clavicipitaceae, Hypocreales), which preys specifically upon them (Fig. 1a). We assessed variation by comparing two host species, Adineta ricciae and A. vaga, which differ by a factor of four in resistance to this pathogen (Fig. 1b). We compared the scale and speed of the transcriptomic response and asked whether horizontally transferred genes were especially likely to be differentially expressed compared to regular metazoan genes in each species. We compared our results with RNA-seq data obtained when bdelloids were exposed to desiccation51, to test whether genes of non-metazoan origin contribute differently when responding to a biotic as opposed to an abiotic stressor. Functional enrichment analysis identified the most strongly upregulated classes of horizontally transferred genes, and revealed groups whose expression profiles and genomic representations differed between the resistant and susceptible rotifer species.
Fig. 1: Response of bdelloid rotifers to inoculation with the fungal pathogen R. globospora.Fig. 1: Response of bdelloid rotifers to inoculation with the fungal pathogen R. globospora. a Active A. ricciae; active and contracted A. vaga; composite of three A. ricciae corpses with fully developed R. globospora infections, differentiating into irregular resting spores and long conidiophores bearing spherical infectious conidia. b Proportion of rotifers killed by infection 72 hours after exposure to R. globospora. Points indicate replicate laboratory populations of A. vaga (n = 16 populations, 189 animals) and A. ricciae (n = 21 populations, 216 animals) exposed to 1000 conidia. Asterisks indicate a highly significant difference in infection mortality (relative risk test, RR = 3.74, 95% CI: 2.8–5.0, z = 8.76, P = 1.4e–25). Boxplots show median and interquartile range (IQR), and whiskers extend to the farthest datapoint from the median that remains within 1.5*IQR of Q1 and Q3 respectively. c Principal component analysis of overall gene expression across A. vaga and A. ricciae orthologous genes. The proportions of total variation accounted for by primary (PC1) and secondary (PC2) components are indicated in parentheses. Clustering of treatment groups across species indicates a strongly shared response in gene expression at T7 that diverges at T24, as A. ricciae moves further and more consistently along the PC1 axis than A. vaga. d Dynamics of gene up- and downregulation relative to control populations inoculated with UV-inactivated spores. Error bars around the observed number of genes in each category show 95% confidence intervals estimated from n = 100 bootstrap replicates (i.e., sampling across all genes with replacement 100 times, each time recalculating the number of shared genes in each category). Asterisks refer to significant differences in the proportion of the species’ respective genesets that are DE (Bonferroni-corrected Chi-square tests, d.f. = 1; n = 58,423 for A. ricciae, 66,273 for A. vaga; P = 9.7e–22, 5.4e–23, 4.7e–38 and 5.8e–8 respectively). e Extent of gene sharing in differentially expressed subsets across timepoints (within species). Values show the number of significantly DE genes shared by intersecting groups. Source data are provided as a Source Data file.
a Active A. ricciae; active and contracted A. vaga; composite of three A. ricciae corpses with fully developed R. globospora infections, differentiating into irregular resting spores and long conidiophores bearing spherical infectious conidia. b Proportion of rotifers killed by infection 72 hours after exposure to R. globospora. Points indicate replicate laboratory populations of A. vaga (n = 16 populations, 189 animals) and A. ricciae (n = 21 populations, 216 animals) exposed to 1000 conidia. Asterisks indicate a highly significant difference in infection mortality (relative risk test, RR = 3.74, 95% CI: 2.8–5.0, z = 8.76, P = 1.4e–25). Boxplots show median and interquartile range (IQR), and whiskers extend to the farthest datapoint from the median that remains within 1.5*IQR of Q1 and Q3 respectively. c Principal component analysis of overall gene expression across A. vaga and A. ricciae orthologous genes. The proportions of total variation accounted for by primary (PC1) and secondary (PC2) components are indicated in parentheses. Clustering of treatment groups across species indicates a strongly shared response in gene expression at T7 that diverges at T24, as A. ricciae moves further and more consistently along the PC1 axis than A. vaga. d Dynamics of gene up- and downregulation relative to control populations inoculated with UV-inactivated spores. Error bars around the observed number of genes in each category show 95% confidence intervals estimated from n = 100 bootstrap replicates (i.e., sampling across all genes with replacement 100 times, each time recalculating the number of shared genes in each category). Asterisks refer to significant differences in the proportion of the species’ respective genesets that are DE (Bonferroni-corrected Chi-square tests, d.f. = 1; n = 58,423 for A. ricciae, 66,273 for A. vaga; P = 9.7e–22, 5.4e–23, 4.7e–38 and 5.8e–8 respectively). e Extent of gene sharing in differentially expressed subsets across timepoints (within species). Values show the number of significantly DE genes shared by intersecting groups. Source data are provided as a Source Data file.
Nowell, R.W., Rodriguez, F., Hecox-Lea, B.J. et al.
Bdelloid rotifers deploy horizontally acquired biosynthetic genes against a fungal pathogen. Nat Commun 15, 5787 (2024). https://doi.org/10.1038/s41467-024-49919-1
Copyright: © 2024 The authors.
Published by Springer Nature Ltd. Open access.
Reprinted under a Creative Commons Attribution 4.0 International license (CC BY 4.0)
By this process, rotifers have existed fundamentally unchanged for hundreds of millions of years.
The mystery for intelligent [sic] design creationists is, why doesn't the putative intelligent designer make greater use of this ability to acquire genes from other organisms instead of repeatedly designing new ways of doing the same thing that we see in almost everything else, and which can better be explained by a mindless, unplanned natural process where no intelligence is involved?
What Makes You So Special? From The Big Bang To You
Ten Reasons To Lose Faith: And Why You Are Better Off Without It




No comments :
Post a Comment
Obscene, threatening or obnoxious messages, preaching, abuse and spam will be removed, as will anything by known Internet trolls and stalkers, by known sock-puppet accounts and anything not connected with the post,
A claim made without evidence can be dismissed without evidence. Remember: your opinion is not an established fact unless corroborated.