Stowers scientists uncover a… | Stowers Institute for Medical Research
"God never restore an amputated limb". This fact, better than anything, illustrates either the limit of the power of prayer and 'faith healing' or the limit of power of the god being prayed to. There are no authenticated examples of a restored limb or of the spontaneous healing of a transected spinal cord, despite the steady supply of victims seeking a cure to 'miracle' site such as Lourdes, and the fervent prayers of victims and the families. (We can dismiss the 'Miracle of Calanda' as far too transparent a hoax to be taken seriously.) It just never happens!
An omniscience, omnipotent, omnibenevolent designer would work to minimise the suffering in its creation. To do otherwise tells us that the alleged creator is:
- Unaware of it, in which case it is not omniscient.
- Aware of it but powerless to prevent it, in which case it is not omnipotent.
- Aware of it but indifferent to it, in which case it is not omnibenevolent.
The facts are not permitted to overrule the sacred dogma - which is creationism in a nutshell.
If you believe in the creationist notion of magic creation, where every species was designed according to some perfect plan, then the fact that some species lack abilities with which other species have, and with which clearly the putative designer knew how to provide, should raise some questions about why the designer made the choice not to provide them.
And if, as most self-centered fundamentalist believe, humans were at the apex of this creator god's design, then it should have the best of everything, and yet there are a myriad of examples of other species being better than humans. (See the AI panel for examples):
Many species have abilities which would be beneficial to humans if we too possessed them* Do you have a list of examples of this? Yes! Many species have evolved unique traits that, if humans possessed them, could greatly enhance our abilities or solve certain challenges. Here's a list of some remarkable examples:These abilities, if adapted for human use, could offer enormous benefits in fields ranging from medicine and survival to transportation and energy efficiency.
- Regeneration (Axolotl, Starfish)
- Benefit: The ability to regrow limbs or even vital organs.
- Species Example: Axolotls and starfish can regenerate entire body parts. This could help humans recover from amputations, spinal cord injuries, or organ damage.
- Echolocation (Bats, Dolphins)
- Benefit: Enhanced navigation and perception in low-visibility conditions.
- Species Example: Bats and dolphins use echolocation to navigate in darkness or murky waters. This would allow humans to navigate dangerous environments or see without light.
- Photosynthesis (Plants, Algae)
- Benefit: The ability to convert sunlight directly into energy.
- Species Example: Plants and algae can photosynthesize, which could eliminate the need for food in some situations, reducing hunger and energy concerns.
- Extreme Durability (Tardigrades)
- Benefit: Surviving extreme environments such as radiation, freezing temperatures, dehydration, and even space.
- Species Example: Tardigrades (water bears) can endure near-death conditions. This would make humans far more resistant to extreme climates or space travel.
- Camouflage (Chameleons, Cuttlefish)
- Benefit: Blending into environments for protection or hunting.
- Species Example: Chameleons and cuttlefish can change color and texture. Humans with this ability could avoid detection in dangerous situations or during combat.
- Electricity Generation (Electric Eel)
- Benefit: Generating electrical energy for self-defense or powering devices.
- Species Example: Electric eels produce electric shocks. This could allow humans to power small devices or defend against threats using natural electricity.
- Hyper-Speed Healing (Deer Antlers, Certain Lizards)
- Benefit: Rapid recovery from wounds and injuries.
- Species Example: Deer regrow antlers each year at an incredibly fast rate. Some lizards regrow tails. Enhanced human healing could lead to faster recovery after surgeries or injuries.
- UV Vision (Birds, Insects)
- Benefit: Seeing ultraviolet light to detect environmental changes or patterns invisible to the human eye.
- Species Example: Many birds and insects can see ultraviolet light, which helps them find food and mates. Humans with this ability could have enhanced perception of their surroundings.
- Extreme Longevity (Greenland Shark, Tortoises)
- Benefit: Significantly extended lifespan.
- Species Example: The Greenland shark can live for centuries, and tortoises often live over 100 years. If humans possessed this trait, we could extend lifespans, allowing more time for knowledge acquisition and creativity.
- Poison Resistance (Mongoose)
- Benefit: Immunity to toxins and venoms.
- Species Example: The mongoose is resistant to snake venom, allowing it to hunt poisonous snakes. This could protect humans from dangerous toxins or make certain medical treatments less risky.
- Hibernation (Bears, Ground Squirrels)
- Benefit: Surviving long periods without food and conserving energy.
- Species Example: Bears and ground squirrels hibernate during the winter. Humans with this ability could endure long periods without food, which would be useful in survival situations or space travel.
- Rapid Adaptation to High Altitudes (Bar-Headed Geese)
- Benefit: Increased oxygen efficiency in high-altitude or low-oxygen environments.
- Species Example: Bar-headed geese can fly over the Himalayas at altitudes that would be deadly to most animals. This would allow humans to thrive in high-altitude environments or survive with less oxygen.
- Flight (Birds, Bats)
- Benefit: Self-powered aerial travel.
- Species Example: Birds and bats have evolved to fly, which could enable humans to travel independently through the air, revolutionizing transportation and exploration.
- Magnetic Field Detection (Migratory Birds, Turtles)
- Benefit: Natural GPS and navigation using Earth’s magnetic fields.
- Species Example: Some birds and turtles can detect the Earth’s magnetic field for navigation during migration. Humans could use this ability for more accurate navigation without technology.
- Temperature Regulation (Camels, Arctic Foxes)
- Benefit: Surviving extreme heat or cold without external aid.
- Species Example: Camels can endure extreme desert heat, and arctic foxes thrive in freezing conditions. Humans with this ability could survive in more hostile climates with ease.
It's in the area of rapid repair and regeneration that research is being concentrated in order to gain a scientific understanding of the processes involved and particularly why they have been lost. One recent example was the discovery that zebra fish can not only repair a transected spinal cord but can regenerate a missing section of their spinal cord and restore it to full functionality.
And now we have another piece of research into how the African killifish can regenerate a missing section of fin, and in particular how the process restores just the missing tissues, no more and no less. This research from the Stowers Institute for Medical Research has just been published, open access, in the Cell Press journal iScience and announced in a Stowers Institute press release:
Stowers scientists uncover a critical component that helps killifish regenerate their fins
The findings are a step toward closing the gap on how we could one day deploy regenerative medicine in humans
Spontaneous injuries like the loss of a limb or damage to the spinal cord are impossible for humans to repair. Yet, some animals have an extraordinary capacity to regenerate after injury, a response that requires a precise sequence of cellular events. Now, new research from the Stowers Institute for Medical Research has unveiled a critical timing factor—specifically how long cells actively respond to injury—involved in regulating regeneration.
A recent study published in iScience on September 20, 2024, sought to understand exactly how an organism knows how much tissue has been lost post-injury. Led by former Predoctoral Researcher Augusto Ortega Granillo, Ph.D., in the lab of Stowers President and Chief Scientific Officer Alejandro Sánchez Alvarado, Ph.D., the team investigated how African killifish properly regrow their tail fin following damage. By analyzing tissue dynamics during regrowth, they found that in addition to known factors, including how many cells are participating and where they are located, the length of time cells spend engaged in the repair process is also key.
One of the greatest unsolved mysteries of regeneration is how an organism knows what has been lost after injury. Essentially, the study points to a new variable in the equation of regeneration. If we can modulate the rate and the length of time that a tissue can launch a regenerative response, this could help us devise therapies that may activate and perhaps prolong the regenerative response of tissues that normally would not do so.
Dr. Alejandro Sánchez Alvarado, senior author.
Stowers Institute for Medical Research,
Kansas City, MO, USA.
Shortly after a killifish tail injury, the remaining tissue needs to know how much damage has occurred. Then, this tissue must enlist the right number of repair cells to the site of injury for the right amount of time. Damage sensing, repair cell recruitment, and timing somehow must work together to regrow the tail.If an animal that can regenerate extremities, like a tail, loses just a tiny portion, how does it know not to regenerate a whole new tail but just the missing piece?
Dr. Alejandro Sánchez Alvarado.
To address this question, the team probed different locations of injury in the killifish tail fin.
They found that skin cells both near an injury and in distant, uninjured regions launch a genetic program that primes the whole animal to prepare for a repair response. Then, skin cells at the site of injury sustain this response and temporarily change their state to modify the surrounding material called the extracellular matrix. Ortega Granillo likens this matrix to a sponge that absorbs secreted signals from the injured tissue that then guides repair cells to get to work. If the signals are not received or not interpreted correctly, the regeneration process may not restore the tail’s original shape and size. Tail fin regeneration at 6 hours after injury. Fluorescence microscopy image shows skin cells (magenta), actively dividing cells (cyan), and transiently activated skin cell states (yellow).
Tail fin regeneration at 6 hours after injury. Fluorescence microscopy image shows skin cells (magenta), actively dividing cells (cyan), and transiently activated skin cell states (yellow).
We very clearly defined when and where—at 24 hours post-injury and in the extracellular matrix—the transient cell state is acting in the fin tissue. Knowing when and where to look allowed us to make genetic disruptions and gain a better understanding of the function of these cell states during regeneration.
Augusto Ortega Granillo, first author
Stowers Institute for Medical Research,
Kansas City, MO, USA.
To investigate whether these distinct cellular states communicate information to the extracellular matrix—the supportive structure surrounding cells—during the repair process, the researchers employed the CRISPR-Cas9 gene editing technique. They specifically targeted a gene known to modify the extracellular matrix, as they had observed its activation at the onset of the regeneration response. By disrupting the function of this gene, the team aimed to determine its role in relaying information from cells to the matrix during regeneration.
These modified animals no longer know how much tissue was lost. They still regenerated, but the speed of tissue growth was deficient. This is telling us that by changing the extracellular space, skin cells inform the tissue how much was lost and how fast it should grow.
Augusto Ortega Granillo.Indeed, the speed and amount of tissue regenerated in these genetically modified killifish increased regardless of whether the tail injury was mild or severe. This finding opens the possibility that cell states that modify the matrix increase regenerative regrowth. If the cell states could be adjusted, it may be a way to stimulate a more robust regeneration response.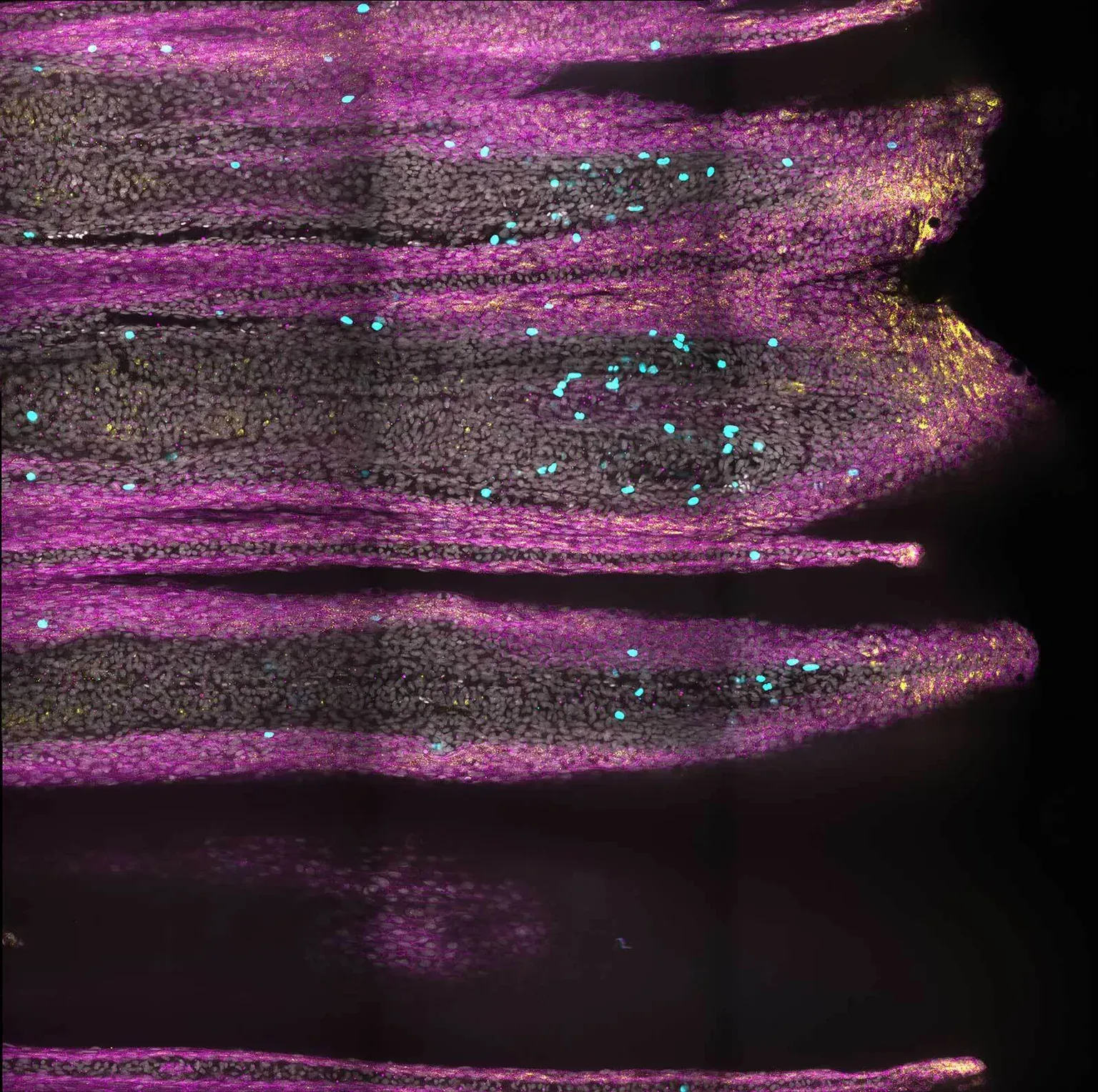 Tail fin regeneration at 7 days post-injury. Fluorescence microscopy image shows skin cells (magenta), actively dividing cells (cyan), and transiently activated skin cell states (yellow), where these cell states are localized now only in the very tip of the fin.
Tail fin regeneration at 7 days post-injury. Fluorescence microscopy image shows skin cells (magenta), actively dividing cells (cyan), and transiently activated skin cell states (yellow), where these cell states are localized now only in the very tip of the fin.
From an evolutionary perspective, understanding why certain organisms excel at regeneration while others, such as humans, have limited regenerative abilities is a driving force in the field of regenerative biology. By identifying general principles in organisms with high regenerative capacity, researchers aim to potentially apply these insights to enhance regeneration in humans. This comparative approach not only sheds light on the evolutionary aspects of regeneration but also holds promise for developing novel therapeutic strategies in regenerative medicine.
Our goal is to understand how to shape and grow tissues. For people who sustain injuries or organ failure, regenerative therapies could restore function that was compromised during illness or following injury.
Augusto Ortega Granillo.Post-injury fin growth shown over time in the African killifish.
Additional authors include Daniel Zamora, Robert Schnittker, Allison Scott, Alessia Spluga, Jonathon Russell, Carolyn Brewster, Eric Ross, Daniel Acheampong, Ning Zhang, Ph.D., Kevin Ferro, Ph.D., Jason Morrison, Boris Rubinstein, Ph.D., Anoja Perera, and Wei Wang, Ph.D.
HighlightsIt's worth repeating what the Sowers Institute news release said at this point:
- Amputation position changes tissue-wide proliferation response
- Regeneration deploys transient regeneration-activated cell states.
- Sqstm1 slows down regenerative outgrowth in distal injuries.
- Prediction: positional information is transduced by ECM changes during regeneration.
Summary
Injury is common in the life of organisms. Because the extent of damage cannot be predicted, injured organisms must determine how much tissue needs to be restored. Although it is known that amputation position affects the regeneration speed of appendages, mechanisms conveying positional information remain unclear. We investigated tissue dynamics in regenerating caudal fins of the African killifish (Nothobranchius furzeri) and found position-specific, differential spatial distribution modulation, persistence, and magnitude of proliferation. Single-cell RNA sequencing revealed a transient regeneration-activated cell state (TRACS) in the basal epidermis that is amplified to match a given amputation position and expresses components and modifiers of the extracellular matrix (ECM). Notably, CRISPR-Cas9-mediated deletion of the ECM modifier sequestosome 1 (sqstm1) increased the regenerative capacity of distal injuries, suggesting that regeneration growth rate can be uncoupled from amputation position. We propose that basal epidermis TRACS transduce positional information to the regenerating blastema by remodeling the ECM.
Introduction
For many organisms, including humans, the preservation of anatomical form and function depends in great part on the periodic elimination and restoration of cells. This process is referred to as tissue homeostasis. In mammals, the rate of tissue homeostasis varies widely across organs; i.e., self-renewal of the intestinal and lung epithelia has been estimated to take 5 days and up to 6 months, respectively.1,2 As such, specific mechanisms exist in adult tissues responsible for sustaining specific rates of tissue homeostasis to mitigate normal, physiological wear and tear. The intricate balance of tissue homeostasis can be severely disrupted by injury. During their lifespans, all multicellular organisms are likely to experience some kind of injury. Unlike physiologically regulated tissue homeostasis, injuries and the extent of the damage incurred are unpredictable. This creates a challenge for organisms to monitor and deploy an anatomically specific regeneration response proportional to the magnitude of the injury.
During regeneration, a specialized structure known as blastema forms through the rapid proliferation and subsequent differentiation of multiple cell types to restore the missing tissue.3 For instance, it has been shown that osteoblast differentiation is accelerated in regenerating fins. Newly differentiated osteoblasts appear 4 days earlier in regenerated tissue compared to the pre-existing tissue, also referred to as the stump.4 It is not known whether there is a regeneration-specific osteoblast differentiation program or if differentiation trajectories used during tissue homeostasis can be accelerated during regeneration. Regeneration has also been shown to alter rates of tissue growth. In vertebrates, the speed of regeneration differs when different amounts of tissue are lost.5 For example, fish caudal fin amputations close to the base of the appendage, a proximal amputation, display higher growth rates than amputations performed further away from the base of the fin, a distal amputation.6,7
To date, it is not known how injured tissues detect amputation position and what processes may encode positional information during regeneration. Efforts to identify deposited positional information prior to injury led to the identification of transcripts and proteins that are differentially expressed along the proximo/distal (P/D) axis in the intact zebrafish fin.8 However, these differences are lost during regeneration, leading to the hypothesis that positional information must be redefined during regeneration.8 Alternatively, it has been proposed that migratory progenitor cells retain positional identity from their locations prior to injury. This opens up the possibility that progenitors communicate positional information to the new tissue to determine regeneration growth rate.9,10 But the mechanism of positional information retention and potential relay to other cells has not yet been identified.
Furthermore, it has been suggested that positional information is encoded in the tissue within the thickness of the bone at the plane of amputation11,12 and that mechanical distension of the epidermis during wound closure constitutes a direct measurement of the amputation position by the wound epidermis. A wave of mechanical distension in the basal epidermis was shown to propagate to different lengths according to the amputation position.13 To add to the complexity of this process, it was shown that there is a 2-day time window at the beginning of regeneration when positional information is possibly reestablished de novo. If blastemas are impaired in their proliferative ability during this time window, the wrong positional information is encoded in the regenerated appendage, so multiple rounds of regeneration consistently grow abnormal tissue sizes in the absence of any further impairment of proliferation.14 It is conceivable that independent mechanisms of positional information coordinating the regenerative response exist. There may be redundant and complementary means to adequately relay and deposit positional information into the new tissue. This would ensure that form and function are restored following diverse and unpredictable injury.
Here, we deploy spatial and temporal analysis of proliferation and single-cell transcriptomic profiling to measure molecular and cellular changes along the caudal fin P/D axis. We chose the African killifish Nothobranchius furzeri for our studies because of the reduced complexity of differential gene expression and gene regulation compared to the more broadly utilized zebrafish.15 We show that amputation position influences the length of time (persistence) it takes for tissues to progress through regeneration. We report on the discovery of a basal epidermal subpopulation that shows a transient regeneration-activated cell state (TRACS) likely to participate in mediating positional information in the regenerating blastema. Altogether, our study demonstrates that amputations along the P/D axis result in defined spatial and temporal rates of proliferation. We propose that such dynamics likely initiate the proportional changes in tissue architecture that may ultimately define the scale and rate of regeneration of amputated tissues.
From an evolutionary perspective, understanding why certain organisms excel at regeneration while others, such as humans, have limited regenerative abilities is a driving force in the field of regenerative biology.
No doubts there then on the part of the authors that the facts can't be explained by evolution and are better explained by creationism's childish superstition, and of course the Theory of Evolution explains it fully without the need to explain why something which evolved for one species or from a common ancestor is retained by one line but not another in terms of the intention, malevolent, benign or indifferent, of the mindless, directionless natural process that drives it.
Creationism, on the other hand, has to try to reconcile the reality with what they believe about their putative designer, which includes explaining why, if it isn't malevolent, it designs organisms to look as though a malevolent designer designed them.




No comments :
Post a Comment
Obscene, threatening or obnoxious messages, preaching, abuse and spam will be removed, as will anything by known Internet trolls and stalkers, by known sock-puppet accounts and anything not connected with the post,
A claim made without evidence can be dismissed without evidence. Remember: your opinion is not an established fact unless corroborated.