Creationism in Crisis
Scientists Now Know How Mammalian Flight Evolved.
No Gods Were Involved
Scientists Now Know How Mammalian Flight Evolved.
No Gods Were Involved
Sugar glider
Creationism in Crisis
Scientists Now Know How Mammalian Flight Evolved.
No Gods Were Involved
Scientists Now Know How Mammalian Flight Evolved.
No Gods Were Involved
Flying Squirrel
Creationism in Crisis
Scientists Now Know How Mammalian Flight Evolved.
No Gods Were Involved
Scientists Now Know How Mammalian Flight Evolved.
No Gods Were Involved
Colugo
Creationism in Crisis
Scientists Now Know How Mammalian Flight Evolved.
No Gods Were Involved
Scientists Now Know How Mammalian Flight Evolved.
No Gods Were Involved
Pipistrelle
Creationism in Crisis
Scientists Now Know How Mammalian Flight Evolved.
No Gods Were Involved
Scientists Now Know How Mammalian Flight Evolved.
No Gods Were Involved
Colugo
Creationism in Crisis
Scientists Now Know How Mammalian Flight Evolved.
No Gods Were Involved
Scientists Now Know How Mammalian Flight Evolved.
No Gods Were Involved
Pipistrelle
Marsupials and other mammals separately evolved flight many times, and we are finally learning how

Sugar glider, Petaurus breviceps
Credit: Simon Stone
Source: Wilderness Society
Source: Wilderness Society
That's nonsense, of course, as gliding mammals and feathered dinosaurs have shown.
Now a team of scientists, led by Dr. Charles Feigin, then of Princeton. University, now of the University of Melbourne, have shown that the genes controlling the development of mammalian powered flight and gliding are common to all mammals, including marsupial gliders and humans, so must have been present in an early common ancestor.
Because no known early mammals had flight, these genes must originally have had some other function and have since been exapted for a different function.
The team's findings were published yesterday, open access, in Science Advances.
The finding is the subject of an article in The Conversation by lead author, Charles Feigin, Postdoctoral Fellow in Genomics and Evolution, The University of Melbourne. His article is reprinted here under a Creative Commons licence, reformatted for stylistic consistency. The original can be read here.

Marsupials and other mammals separately evolved flight many times, and we
are finally learning how
Anom Harya/Shutterstock
Charles Feigin, The University of Melbourne
Shoot for the moon. Even if you miss, you’ll land on the next tree. Many groups of mammals seem to have taken this evolutionary advice to heart. According to our newly published paper in Science Advances, unrelated animals may even have used the same blueprints for building their “wings”.
While birds are the undisputed champions of the sky, having mastered flight during the Jurassic, mammals have actually evolved flight more often than birds. In fact, as many as seven different groups of mammals living today have taken to the air independently of each other.
These evolutionary experiments happened in animals scattered all across the mammalian family tree – including flying squirrels, marsupial possums and the colugo (cousin of the primates). But they all have something in common. It’s a special skin structure between their limbs called a patagium, or flight membrane.
The fact these similar structures have arisen so many times (a process called convergent evolution) hints that the genetic underpinnings of patagia might predate flight. Indeed, they could be shared by all mammals, even those living on the ground.
If this is true, studying patagia can help us to better understand the incredible adaptability of mammals. We might also discover previously unknown aspects of human genetics.

Sugar gliders are one of several mammals that have independently evolved
the ability to fly through the air.
apiguide/Shutterstock
Despite being seemingly simple skin structures, patagia contain several tissues, including hair, a rich array of touch-sensitive neurons, connective tissue and even thin sheets of muscle. But in the earliest stages of formation, these membranes are dominated by the two main layers of the skin: the inner dermis and outer epidermis.
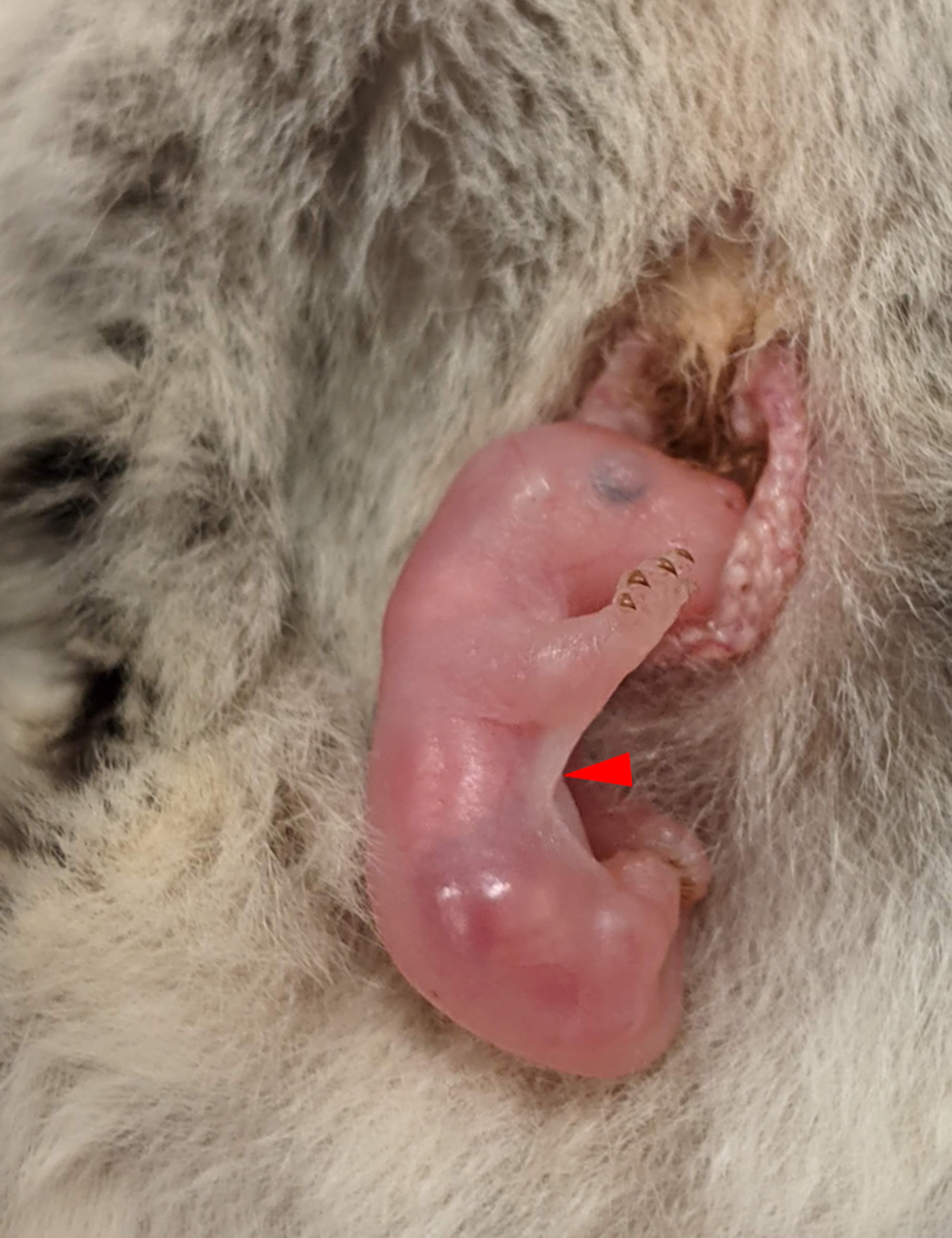
The patagium in sugar gliders (red arrow) forms after birth when the
newborn, or joey, is in its marsupial mother’s pouch.
Charles Feigin, Author provided
In some mammals, this differentiation happens when they are still an embryo in the uterus. Incredibly though, in our main model species – the marsupial sugar glider (Petaurus breviceps) – this process begins after birth, while they are in the mother’s pouch. This provides us with an incredible window into patagium formation.
Starting with the sugar glider, we examined the behaviours of thousands of genes active during the early development of the patagium, to try and div out how this chain of events is kicked off.
From gliders to bats
We discovered that levels of a gene called Wnt5a are strongly correlated with the onset of those early skin changes – condensation and hyperplasia. Through a series of experiments involving cultured skin tissues and genetically engineered laboratory mice, we showed that adding extra Wnt5a was all it took to drive both of these early hallmarks of patagium formation.
Interestingly, when we extended our work to bats, we found extremely similar patterns of Wnt5a activity in their developing lateral patagia to that in sugar gliders. This was surprising, since bats (placental mammals) last shared a common ancestor with the marsupial sugar glider around 160 million years ago.
Perhaps even more remarkably, we found a nearly identical pattern in the outer ear (or pinna) of lab mice. The pinna is a nearly universal trait among mammals, including innumerable species with no flying ancestry.

Seba’s short-tailed bat has a lateral patagium (connected to the flank of
the body) activated by Wnt5a.
Together, these results suggest something profound. Wnt5a’s role in ushering in the skin changes needed for a patagium likely evolved long before the first mammal ever took to the air.
Originally, the gene had nothing to do with flight, instead contributing to the development of seemingly unrelated traits. But because of shared ancestry, most living mammals today inherited this Wnt5a-driven program. When species like gliders and bats started on their separate journeys into the air, they did so with a common “molecular toolkit”.
Not only that, but this same toolkit is likely present in humans and working in ways we don’t fully understand yet.
There are definite limits to our recent work. First, we haven’t made a flying mouse. This may sound like a joke, but demonstrates we still don’t fully understand how a region of dense, thick skin becomes a thin and wide flight membrane. Many more genes with unknown roles are bound to be involved.
Second, while we’ve shown a cause-and-effect relationship between Wnt5a and patagium skin differentiation, we don’t know precisely how Wnt5a does it. Moving forward, we hope to fill in these gaps by broadening the horizons of our cross-species comparisons and by conducting more in-depth molecular studies on patagium formation in sugar gliders.
For now though, our study presents an exciting new view of flight in mammals. We may not be the strongest fliers, but trying is in our DNA.
This article is republished from The Conversation under a Creative Commons license. Read the original article.
 Copyright: © 2023 The authors.
Copyright: © 2023 The authors.Published by American Association for the Advancement of Science.
Open access. (CC BY 4.0)
AbstractAnd another unintelligently designed argument for intelligent design creationism lies in ruins, having failed to fly, leaving Creationists to explain why all mammals. including humans, have the genes that are used to develop the mechanism for flight in several different orders of mammals.
Lateral flight membranes, or patagia, have evolved repeatedly in diverse mammalian lineages. While little is known about patagium development, its recurrent evolution may suggest a shared molecular basis. By combining transcriptomics, developmental experiments, and mouse transgenics, we demonstrate that lateral Wnt5a expression in the marsupial sugar glider (Petaurus breviceps) promotes the differentiation of its patagium primordium. We further show that this function of Wnt5a reprises ancestral roles in skin morphogenesis predating mammalian flight and has been convergently used during patagium evolution in eutherian bats. Moreover, we find that many genes involved in limb development have been redeployed during patagium outgrowth in both the sugar glider and bat. Together, our findings reveal that deeply conserved genetic toolkits contribute to the evolutionary transition to flight in mammals.
Feigin, Charles Y.; Moreno, Jorge A.; Ramos, Raul; Mereby, Sarah A.; Alivisatos, Ares; Wang, Wei; van Amerongen, Renée; Camacho, Jasmin; Rasweiler, John J.; Behringer, Richard R.; Ostrow, Bruce; Plikus, Maksim V.; Mallarino, Ricardo
Convergent deployment of ancestral functions during the evolution of mammalian flight membranes
Science Advances 9(12), eade7511. DOI: 10.1126/sciadv.ade7511
Copyright: © 2023 The authors.
Published by American Association for the Advancement of Science. Open access.
Reprinted under a Creative Commons Attribution 4.0 International license (CC BY 4.0)

No comments :
Post a Comment
Obscene, threatening or obnoxious messages, preaching, abuse and spam will be removed, as will anything by known Internet trolls and stalkers, by known sock-puppet accounts and anything not connected with the post,
A claim made without evidence can be dismissed without evidence. Remember: your opinion is not an established fact unless corroborated.