Religion, Creationism, evolution, science and politics from a centre-left atheist humanist. The blog religious frauds tell lies about.
Wednesday, 27 November 2024
Mallevolent Design - How Salmonella Sneaks Past Our Defences To Make Us Sick
New study shows how salmonella tricks gut defenses to cause infection
There is a simple paradox at the heart of creationism that I have never even seen an attempt to resolve. It all comes from two beliefs: there is only one designer god capable of designing living organisms and that designer god designed us complete with our immune system with which we can attempt to resist attack by pathogens, and that pathogens are not the work of this design, but are the result of 'genetic entropy' and 'devolution' since Adam & Eve let 'sin' into the world. The fact that Michael J. Behe, who invented that excuse, has let slip that ID Creationism is Bible literalism in a lab coat seems to be lost on his followers who still dutifully insist that it is a scientific alternative to evolution and should be taught in school science class (presumably now with the tale of Adam & Eve taught as real history and 'sin' as a real force in science).
The paradox is, did the designer god give Adam & Eve an immune system, or did it design an upgrade when 'sin' allowed pathogens to exist? If the former, it was anticipating and planning for the so-called 'fall'; if the latter, it lacked foresight so is not omniscient.
But however creationists resolve this paradox they still have to explain why the 'intelligently designed' immune system doesn't work very well and why whatever is designing pathogens seems to be able to overcome it.
The nonsense about 'sin', 'the fall', etc., is trivially easy to refute because any improvement in a parasite's ability to parasitise its host can't possibly be regarded as a devolution from some assumed initial perfection because an improvement can't be worse that what it's an improvement on. The whole nonsense of 'devolution' is biological gobbledygook, intelligently designed to appeal to scientifically illiterate simpletons who want to fit the Bible superstition somewhere in the reasoning without bothering too much about the logic or the biology.
So, the paradox boils down to why an intelligent designer would be having an arms race with itself so the parasites it creates can continue to parasitise the victims it creates complete with their immune system it created to stop them. Creationists normally flee in terror at the mere mention of arms races, which is why you'll never see them discussed in the cult literature apart from where pathogens are waved aside as 'caused by sin', blah, blah, blah...
So, it would be refreshing indeed to see a genuine attempt by an intelligent design creationist try to give some rational explanation, and hopefully without giving away the fact that ID creationism is merely Christian fundamentalism in disguise, for the discovery by a new UC Davis Health study that shows how the Salmonella bacteria, a major cause of food poisoning, can invade the gut even when protective bacteria are present.
As an added embarrassment for creationists, Salmonella is closely related to Escherichia coli (E.coli) that they usually cite Michael J. Behe as 'proving' it must have been designed by their god because its flagellum is 'irreducibly complex'.
First a little AI background information about Salmonella, where it came from and what it does to us:
Tuesday, 26 November 2024
Transitional Form News - Precambrian Common Ancestor of Insects, Arachnids, and Nematode Worms

The refutation of creationism continues today with news of another one of those 'non-existent' transitional species that turn up with monotonous regularity only to be dismissed by creationists as 'not transitional but fully formed' with now two gaps in the record where there was originally one, or by simply dismissing the dating method as unreliable and coincidentally wrong by an order of magnitude sufficient to make 6-10,000 years look like x-million years.
Another big disappointment for creationists is the fact that this one is from before the Cambrian when their traditional disinformation claims lots of species popped into existence without ancestors by magic in a single event called the 'Cambrian explosion'. The Cambrian 'explosion' was of course a period of some 6-10 million years during which many of the basic body plans of multicellular organisms evolved.
This fossil however was before then and was clearly the ancestral stem species from which a whole range of Cambrian organism, collectively known as Ecdysozoa evolved. These are a group of organisms with an outer cuticle which is shed periodically as the organism grows. The vast group includes nematode worms and arthropods such as insects, spiders, crustaceans like crabs, shrimps, lobsters, and the horseshow crab. So, this discovery, which the palaeontologists have named Uncus dzaugisi sits at the base of this branch of the evolutionary tree. It resembled a nematode worm.
Tell me all about the Ecdysozoa, please. Ecdysozoa is a major clade of animals within the larger group known as Protostomia, distinguished by their unique mode of growth, which involves periodic moulting of an external cuticle. The term "ecdysozoa" comes from the Greek word ecdysis, meaning "to strip off" or "to shed," referring to this moulting process.The discovery was made by a team from University of California, Riverside (UCR), led by Professor Mary Droser a distinguished professor of geology. The have explained their findings in the journal, Current Biology, and in a UCR press release:
Characteristics of Ecdysozoa
- Moulting (Ecdysis):
- The defining feature of Ecdysozoans is the shedding of their cuticle—a tough, non-living outer layer made of proteins, polysaccharides (like chitin), or collagen. This process allows them to grow, as the rigid cuticle limits continuous size increase.
- After moulting, a new, larger cuticle is secreted, which hardens over time.
- Body Structure:
- Many have a hydrostatic skeleton, relying on fluid pressure in their body cavity for movement and structure.
- Most lack cilia or flagella in their adult form, distinguishing them from other protostomes.
- Diversity:
- The group includes some of the most diverse and abundant organisms on Earth, ranging from microscopic species to large, visible ones.
- Nervous System:
- Typically, they have a ventral nerve cord and a dorsal brain, with varied sensory adaptations.
Major Groups Within Ecdysozoa
Ecdysozoa includes eight phyla, with two of the most well-known being Arthropoda and Nematoda:
- Arthropoda
- The largest and most diverse group of animals, including insects, arachnids, crustaceans, and myriapods.
- They have segmented bodies, jointed appendages, and a hard exoskeleton.
- Examples: Butterflies, crabs, spiders, centipedes.
- Nematoda (Roundworms)
- Microscopic to macroscopic worms with a cylindrical, unsegmented body.
- Found in nearly every habitat on Earth, often as parasites in plants and animals.
- Examples: Caenorhabditis elegans (a model organism), Ascaris (a human intestinal parasite).
- Tardigrada (Water Bears)
- Microscopic, water-dwelling animals known for their extreme resilience to harsh environments.
- They can survive desiccation, radiation, and even the vacuum of space.
- Onychophora (Velvet Worms)
- Segmented, soft-bodied terrestrial animals with slime glands used for capturing prey.
- Priapulida
- Marine worms with a tubular body, often found burrowing in sediment.
- Kinorhyncha
- Tiny, segmented marine animals often referred to as "mud dragons."
- Loricifera
- Microscopic animals inhabiting marine sediment, with a complex, retractable body.
- Nematomorpha (Horsehair Worms)
- Parasites as larvae, often controlling the behaviour of their insect hosts.
Evolution and Phylogeny
- Ecdysozoa was proposed as a clade in 1997, based on molecular evidence (notably studies of ribosomal RNA).
- They share a common ancestor with other protostomes but diverged early to adopt their unique moulting strategy.
- Their evolutionary success is evident in their sheer numbers and adaptability, especially in arthropods.
Ecological and Economic Importance
- Ecological Roles:
- Many ecdysozoans are key components of ecosystems as decomposers, predators, prey, and parasites.
- Insects, a subgroup of arthropods, are critical for pollination and nutrient cycling.
- Economic Impact:
- Beneficial: Insects (e.g., bees) aid in agriculture, while nematodes play a role in soil health.
- Harmful: Some ecdysozoans are pests or parasites causing diseases in humans, animals, and crops.
Fun Facts
- Tardigrades can enter a state called cryptobiosis, surviving for decades without water or food.
- Arthropods are the most successful phylum, with over a million described species, and likely many more undiscovered.
- Nematodes may account for about 80% of individual animals on Earth.
Tiny worm makes for big evolutionary discovery
UC Riverside scientists have described ‘Uncus,’ the oldest ecdysozoan and the first from the Precambrian period
Everyone has a past. That includes the millions of species of insects, arachnids, and nematode worms that make up a major animal group called the Ecdysozoa. Until recently, details about this group’s most distant past have been elusive. But a UC Riverside-led team has now identified the oldest known ecdysozoan in the fossil record and the only one from the Precambrian period. Their discovery of Uncus dzaugisi, a worm-like creature rarely over a few centimeters in length, is described in a paper published today in Current Biology.
Scientists have hypothesized for decades that this group must be older than the Cambrian, but until now its origins have remained enigmatic. This discovery reconciles a major gap between predictions based on molecular data and the lack of described ecdysozoans prior to the rich Cambrian fossils record and adds to our understanding of the evolution of animal life.
Mary L. Droser, co-author Earth and Planetary Sciences University of California, Riverside
Riverside, CA , USA.
The ecdysozoans are the largest and most species-rich animal group on Earth, encompassing more than half of all animals. Characterized by their cuticle — a tough external skeleton that is periodically shed — the group comprises three subgroups: nematodes, which are microscopic worms; arthropods, which include insects, spiders, and crustaceans; and scalidophora, an eclectic group of small, scaly marine creatures.Like many modern-day animal groups, ecdysozoans were prevalent in the Cambrian fossil record and we can see evidence of all three subgroups right at the beginning of this period, about 540 million years ago. We know they didn’t just appear out of nowhere, and so the ancestors of all ecdysozoans must have been present during the preceding Ediacaran period.
Ian V. Hughes, first author
Organismic and Evolutionary Biology
Harvard University, Cambridge, MA, USA.
DNA-based analyses, used to predict the age of animal groups by comparing them with their closest living relatives, have corroborated this hypothesis. Yet ecdysozoan fossil animals have remained hidden among scores of animal fossils paleontologists have discovered from the Ediacaran Period.
Ediacaran animals, which lived 635-538 million years ago, were ocean dwellers; their remains preserved as cast-like impressions on the seabed that later hardened to rock. Hughes said uncovering them is a labor-intensive, delicate process that involves peeling back rock layers, flipping them over, dusting them off, and piecing them back together to get “a really nice snapshot of the sea floor.” Top: Uncus fossil from Nilpena Ediacara National Park. The numbers correspond to the coordinates of this fossil on the fossil bed surface. Bottom: 3D laser scans enable the researchers to study the fossils’ shape and curvature.Droser Lab/UCR.
Top: Uncus fossil from Nilpena Ediacara National Park. The numbers correspond to the coordinates of this fossil on the fossil bed surface. Bottom: 3D laser scans enable the researchers to study the fossils’ shape and curvature.Droser Lab/UCR.
This excavation process has only been done at Nilpena Ediacara National Park in South Australia, a site Droser and her team have been working at for 25 years that is known for its beautifully preserved Ediacaran fossils.
Nilpena is perhaps the best fossil site for understanding early animal evolution in the world because the fossils occur during a period of heightened diversity and we are able to excavate extensive layers of rock that preserve these snapshots. The layer where we found Uncus is particularly exciting because the sediment grains are so small that we really see all the details of the fossils preserved there.
Assistant Professor Scott Evans, co-author
Earth, Ocean, and Atmospheric Sciences
Florida State University, Tallahassee, FL, USA.
While the team didn’t set out to find an early ecdysozoan during their 2018 excavation, they were drawn to a mysterious worm-like impression that they dubbed “fishhook.”
Sometimes we make dramatic discoveries and sometimes we excavate an entire bed and say ‘hmmm, I’ve been looking at that thing, what do you think?’ That’s what happened here. We had all sort of noticed this fishhook squiggle on the rock. It was pretty prominent because it was really, really deep.
Because it was deep, we knew it wasn’t smooshed easily so it must have had a pretty rigid body. At this point we knew this was a new fossil animal and it belong to the Ecdysozoa.
Ian V. Hughes
After seeing more of the worm-like squiggles the team paid closer attention, taking note of fishhook’s characteristics. Other defining characteristics include its distinct curvature and the fact that it could move around — seen by trace fossils in the surrounding area. Paul De Ley, an associate professor of nematology at UCR, confirmed its fit as an early nematode and ruled out other worm types.
The team called the new animal Uncus, which means “hook” in Latin, noting in the paper its similarities to modern-day nematodes. Hughes said the team was excited to find evidence of what scientists had long predicted; that ecdysozoans existed in the Ediacaran Period.
It’s also really important for our understanding of what these early animal groups would have looked like and their lifestyle, especially as the ecdysozoans would really come to dominate the marine ecosystem in the Cambrian.
Ian V. Hughes
The paper is titled “An Ediacaran bilateran with an ecdysozoan affinity from South Australia.” Funding for the research came from NASA.
HighlightsI think my favourite quote from one of the scientists is "We know they didn’t just appear out of nowhere, and so the ancestors of all ecdysozoans must have been present during the preceding Ediacaran period", which just about describes the difference between someone who knows the Theory of Evolution is correct because he understands the evidence for it, and a creationists who believes in fully formed living organisms made from nothing, magically popping into existence from nowhere, with magic spells cast by an unproven supernatural deity their mummy and daddy told them about.
- A new, motile bilaterian is described from the Ediacaran of South Australia
- Features including morphology and movement suggest an ecdysozoan affinity
- This discovery firmly places ecdysozoans in the Precambrian
Summary
Molecular clocks and Cambrian-derived metazoans strongly suggest a Neoproterozoic origin of many animal clades.1,2,3,4 However, fossil bilaterians are rare in the Ediacaran, and no definitive ecdysozoan body fossils are known from the Precambrian. Notably, the base of the Cambrian is characterized by an abundance of trace fossils attributed to priapulid worms,5,6 suggesting that major divisions among ecdysozoan groups occurred prior to this time. This is supported by ichnofossils from the latest Ediacaran or early Cambrian left by a plausible nematoid,7,8,9 although definitively attributing this inferred behavior to crown-Nematoida remains contentious in the absence of body fossils.10 Given the high probability of the evolution of Ecdysozoa in the Proterozoic, the otherwise prolific fossil record of the Ecdysozoa, and the identification of more than 100 distinct Ediacaran genera, it is striking that no Ediacaran body fossils have been confidently assigned to this group. Here, we describe Uncus dzaugisi gen. et. sp. nov. from the Ediacara Member (South Australia), a smooth, vermiform organism with distinct curvature and anterior-posterior differentiation. The depth of relief of Uncus is unique among Ediacara fossils and consistent with a rigid outer cuticle. Ecological relationships and associated trace fossils demonstrate that Uncus was motile. Body morphology and the inferred style of movement are consistent with Nematoida, providing strong evidence for at least an ecdysozoan affinity. This validates the Precambrian origin of Ecdysozoa, reconciling a major gap between predicted patterns of animal evolution and the fossil record.4
Hughes, Ian V.; Evans, Scott D.; Droser, Mary L.
An Ediacaran bilaterian with an ecdysozoan affinity from South Australia
Current Biology, DOI: 10.1016/j.cub.2024.10.030
© 2024 Elsevier.
Reprinted under the terms of s60 of the Copyright, Designs and Patents Act 1988.
The ancestral form, the transitional species, was in exactly for rock formation of exactly the right age which the theory of evolutionary decent with modifiction from a common ancester predicted.
And in case a creationist is tempted to try the 'radiometric dating is flawed/wrong/faked fallacy. The Ediacaran rock formation these fossils were found in was independently dated several different ways that all converged on a 98-million-year span from 635 to 538 million years ago known as the Ediacaran. The Most important being the Uranium-Lead (U-Pb) dating of zircons found in the layers of volcanic ash sandwiched within the rocks. To compress 600 million years of radioactive decay into less than 6-10,000 years would have caused Earth's rocks to melt and the seas to boil away. And the weak nuclear force would have been so weak that atoms could not have formed, let alone life, and there would have been no planet and no universe to fine tune for it either.
Refuting Creationism: Why Creationism Fails In Both Its Science And Its Theology
The Failure of Creationism: The Theory That Never Was
Common Ancestry - Ancient Choanoflagellates Genes Used To Make A Mouse
Scientists recreate mouse from gene older than animal life - Queen Mary University of London
If there is one thing designed to get creationists chanting 'Common Designer!' it's evidence of the same gene doing the same thing in lots of different organisms, no matter how distantly related they are.
But when that gene is needed because of a basic design blunder long ago in the evolution of multicellular organisms, that chant looks increasingly forlorn.
For example, scientists have just shown how SOX and POU genes isolated from a single-celled choanoflagellate can be used to convert a mouse cell to function as the stem cell to clone another mouse. The reason this works is because something needs to reset the epigenetic setting in specialised cells. In a multicellular organism like a mouse, this effectively means any cell produced after the first few cell divisions of the fertilised zygote.
But why would a single-celled organism like a choanoflagellate need to do that? The answer it that epigenetics originally evolved to make an organism more able to respond to environmental changes and stresses.
Sunday, 24 November 2024
Refuting Creationism - How Pterosaurs Evolved
New fossil discovery reveals key step in the evolution of flying reptiles - Queen Mary University of London
Although creationists insist dinosaurs coexisted with humans before their god launched his genocidal flood, and even twist words like 'behemoth' in the Bible to try to show how the authors of genesis were familiar with dinosaurs (as though there was only one species) what they never explain is why the same authors said nothing at all about the pterodactyls that were contemporaneous with dinosaurs.
The real reason is of course that pterodactyls and dinosaurs lived in that vast expanse of time before 'Creation Week' when 99.9975% of everything that happened on Earth happened.
The evidence for this can be found in any palaeontological article that deals with the evolution of these first flying vertebrates, like this one about the discovery of a new species, Skiphosoura bavarica, which is also helping to understand how these reptiles evolved. It was identified by a team led by Dr David Hone, a palaeontologist from Queen Mary University of London. Early pterosaurs had a wingspan of about 2 meters (6 feet) but they evolved into massive creatures with wing spans up to 10 meters (30 feet). On the ground, some of them may have been at tall as a giraffe!
Pterosaurs, their evolution, and their relationship to dinosaurs. Pterosaurs were a group of flying reptiles that lived during the Mesozoic Era, from the late Triassic (about 228 million years ago) to the end of the Cretaceous (66 million years ago). They were the first vertebrates to achieve powered flight and were remarkable for their diversity in size, morphology, and ecological niches. Here's a detailed overview of pterosaurs, their evolution, and their relationship to dinosaurs:Dr Hone's team have published their findings in the journal Current Biology and describe it in a Queen Mary University news release:
- Evolutionary Origins
- Pterosaurs belonged to the clade Pterosauria, which is part of the larger group Archosauria, making them close relatives of dinosaurs and crocodilians.
- Their exact evolutionary origins are debated, but they likely evolved from small, ground-dwelling or arboreal reptiles within the clade Avemetatarsalia, which also includes dinosaurs and birds.
- Early pterosaurs, such as Eudimorphodon and Dimorphodon, appeared in the late Triassic and already exhibited the characteristic wing structure.
- Anatomy and Adaptations for Flight
- Pterosaurs' wings were formed by a membrane of skin, muscle, and other tissues stretched along an elongated fourth finger, which supported the main wing structure.
- Other adaptations included:
- Lightweight skeletons with hollow bones to reduce weight.
- Keel-like breastbones to anchor powerful flight muscles.
- Complex cranial crests in some species, possibly for display or aerodynamic purposes.
- Unique respiratory adaptations with air sacs similar to those in modern birds.
- Relationship to Dinosaurs
- Pterosaurs and dinosaurs share a common ancestor, but they are distinct groups within Archosauria. Pterosaurs are not considered dinosaurs.
- The distinction lies in their lineage: dinosaurs belong to the clade Dinosauria, while pterosaurs form their own separate clade.
- Diversity and Evolutionary Trends
- Pterosaurs diversified into two main groups:
- Rhamphorhynchoids (Early Pterosaurs):
- Typically small to medium-sized.
- Long tails with a vane or rudder-like structure at the tip.
- Examples: Rhamphorhynchus, Dimorphodon.
- Lived during the Triassic and Jurassic periods.
- Pterodactyloids (Advanced Pterosaurs):
- Larger body sizes, including giants like Quetzalcoatlus with wingspans exceeding 10 meters.
- Short tails or no tails.
- Adapted to various ecological roles, such as fish-eating, filter-feeding, and scavenging.
- Examples: Pteranodon, Istiodactylus, Quetzalcoatlus.
- Dominated the skies during the Jurassic and Cretaceous periods.
Ecological Roles
- Pterosaurs occupied diverse ecological niches:
- Some were piscivores, using long, pointed jaws to catch fish.
- Others were filter feeders, like Pterodaustro, which had bristle-like teeth.
- Large pterosaurs may have been scavengers or predators of small terrestrial animals.
- Their crests may have been used for sexual selection, thermoregulation, or species recognition.
Extinction
- Pterosaurs went extinct at the end of the Cretaceous period during the mass extinction event 66 million years ago, likely caused by the asteroid impact and subsequent environmental changes.
- Birds, which evolved from theropod dinosaurs, survived and continued to dominate the skies, taking over many of the ecological niches once held by pterosaurs.
Fossil Evidence
- Pterosaur fossils are relatively rare due to their fragile skeletons, but significant discoveries have been made worldwide, revealing exquisite details of their anatomy and even soft tissues like wing membranes and pycnofibers (hair-like structures covering their bodies).
Key Distinctions from Dinosaurs
- Dinosaurs were primarily terrestrial, with a diverse range of locomotion and body plans, whereas pterosaurs were adapted for flight.
- Birds are considered modern-day dinosaurs (descendants of theropods), but they are not descended from pterosaurs.
Pterosaurs are an incredible example of evolutionary innovation, showcasing how vertebrates conquered the skies long before birds. Their fossils continue to provide insights into the complexity of prehistoric life and the adaptive potential of ancient reptiles.
New fossil discovery reveals key step in the evolution of flying reptiles
A remarkable new fossil discovery is shedding light on how flying reptiles, known as pterosaurs, evolved from their early forms into the later giants that ruled prehistoric skies.
The new species, named Skiphosoura bavarica, was identified by a team led by Dr David Hone, a palaeontologist from Queen Mary University of London. Their findings were published today in the journal Current Biology.
The pterosaurs, close relatives of dinosaurs, were the first vertebrates to achieve powered flight. While early species typically had wingspans of about 2 metres, later pterosaurs evolved into enormous forms with wingspans reaching 10 metres. The discovery of Skiphosoura bavarica provides critical insights into how these transformations occurred.
Hailing from southern Germany, Skiphosoura boasts a rare, nearly complete skeleton preserved in three dimensions—a significant contrast to the often-flattened fossils of its relatives. Measuring about 2 metres in wingspan, the new species’ most striking feature is its short, stiff, sword-like tail, which inspired its name: “sword tail from Bavaria.”For two centuries, scientists divided pterosaurs into two major groups: early non-pterodactyloids, characterised by short heads, long tails, and specific wing and toe structures, and the later pterodactyloids, which had larger heads, shorter tails, and other adaptations for efficient flight. Intermediate species, like the Darwinopterus discovered in the 2010s, showed how the head and neck evolved first. Skiphosoura represents a critical step beyond the Darwinopterus. Its head and neck resemble the more advanced pterodactyloids, while its wrist, tail, and foot show transitional features. These traits help trace the gradual adaptations that allowed later pterosaurs to grow to massive sizes. The study also reconstructed the evolutionary family tree of pterosaurs, placing Skiphosoura between Darwinopterus and true pterodactyloids. Additionally, a Scottish pterosaur named Dearc was identified as a key intermediate between early pterosaurs and Darwinopterus. Together, these findings form a near-complete evolutionary sequence for pterosaurs, detailing how their anatomy changed over time. The discovery was made possible through the efforts of an international team. Adam Fitch, from the University of Wisconsin-Madison, highlighted the significance of Skiphosoura:This is an incredible find. It really helps us piece together how these amazing flying animals lived and evolved. Hopefully, this study will inspire more research into this important evolutionary transition.
Dr. David William Elliott Hone, lead author, School of Biological and Behavioural Sciences
Queen Mary University of London, UK.
Pterosaurs have long been symbols of the unique life of the past. Skiphosoura represents an important new form for working out pterosaur evolutionary relationships and how this lineage arose and changed.
Adam Fitch, co-author
University of Wisconsin-Madison UW Geology Museum, Madison, WI, USA.Having worked on over 60 pterosaurs from the Solnhofen limestone, it became clear during preparation that this fossil displayed features from both major groups of pterosaurs, with the shortened tail being a crucial diagnostic trait.
Stefan Selzer, co-author
Grabenäcker 12, Hemhofen, Germany.
Bruce and René Lauer of the Lauer Foundation, who contributed to the project, underscored the importance of modern techniques such as UV photography in uncovering fine details of the specimen.
We are proud to bring this important specimen to science and further the understanding of pterosaur evolution.
Bruce Lauer, co-author
Lauer Foundation for Paleontology, Science and Education
Wheaton, IL, USA.
With its blend of cutting-edge research, meticulous preparation, and international collaboration, the study of Skiphosoura bavarica offers a significant leap forward in understanding the evolutionary journey of these extraordinary flying reptiles.
HighlightsAs Though to rub salt into creationist wounds, not only is this pterosaur from the Lower Tithonian (i.e. 148-150.8 million years old) but as the palaeontologist explain, it forms part of a transitional sequence of fossils showing how this group of reptiles evolved.Summary For over a century, there was a major gap in our understanding of the evolution of the flying Mesozoic reptiles, the pterosaurs, with a major morphological gap between the early forms and the derived pterodactyloids.1 Recent discoveries have found a cluster of intermediate forms that have the head and neck of the pterodactyloids but the body of the early grade,2 yet this still leaves fundamental gaps between these intermediates and both earlier and more derived pterosaurs. Here, we describe a new and large Jurassic pterosaur, Skiphosoura bavarica gen. et sp. nov., preserved in three dimensions, that helps bridge the gap between current intermediate pterosaurs and the pterodactyloids. A new phylogeny shows that there is a general progression of key characteristics of increasing head size, increasing length of neck and wing metacarpal, modification to the fifth toe that supports the rear wing membrane, and gradual reduction in tail length and complexity from earlier pterosaurs into the first pterodactyloids. This also shows a clear evolution of the increasing terrestrial competence of derived pterosaurs. Furthermore, this closes gaps between the intermediates and their ancestors and descendants, and it firmly marks the rhamphorhynchines and ctenochasmatid clades as, respectively, being the closest earliest and latest groups to this succession of transitional forms.
- A new pterosaur, Skiphosoura bavarica, is named from the Jurassic of Germany
- The specimen is much larger than other known forms and is preserved in three dimensions
- The Skiphosoura helps document the transition from early pterosaurs to the pterodactyloids
- The tail is short but retains the supporting structures of earlier forms
 Figure 1 Key elements of Skiphosoura
Figure 1 Key elements of Skiphosoura
ca, caudal vertebra; cn, chevron; dr, dorsal rib; fe, femur; hu, humerus; pb, pubis; pm, premaxilla; wpx 1, wing phalanx 1. See also Figures S1, S2, S7, and S9
- The anterior part of the skull with the premaxillary crest and large teeth. Also seen is the humerus. Scale bar, 100 mm.
- The short caudal vertebrate with long zygapophyses and the elongate chevrons. Scale bar is 10 mm.
 Figure 2 Simplified phylogeny of Macronychoptera showing the phylogenetic placement of Skiphosoura bavarica gen. et sp. nov
Figure 2 Simplified phylogeny of Macronychoptera showing the phylogenetic placement of Skiphosoura bavarica gen. et sp. nov
The new taxon is recovered outside of Pterodactyloidea as a late-diverging member of a grade of non-pterodactyloid monofenestratans. Note that Monofenestrata also lies within an earlier-diverging grade of “rhamphorhynchids” (Dorygnathus to Angustinaripterus) and that “rhamphorhynchines” (Rhamphorhynchini, Dearc, Angustinaripterus) here represent the closest relatives of Monofenestrata. See also Figure S10 and Table S2. Figure 3 The transition of pterosaur proportions across the transition from early pterosaurs to the pterodactyloids
Figure 3 The transition of pterosaur proportions across the transition from early pterosaurs to the pterodactyloids
Upper left: Nopsca curves of the proportions of major elements of the skeleton scaled against the length of the humerus showing the transition of major proportions. Center: simplified phylogeny showing the transition of key characters in the evolution of pterodactyloids from the rhamphorhynchines and “through” the early monofenestratans. Skeletals showing the transition with representational taxa: Rhamphorhynchus, Dearc, Darwinopterus, Skiphosoura, and Pterodactylus. This shows the transitions of multiple features across the tree: (1) the increasing length of the skull and increase of the size of the naris before fusion to form the NAOF, (2) increase in the length of the cervical series, (3) proportional reduction in the length of the wingfinger and increase in the length of the proximal wing, (4) increase in the length of the first wing phalanx to be the longest of the four phalanges, (5) increase in the length of the wing metacarpal, (6) increase in the size of the prepubes, (7) reduction of the fifth toe, and (8) reduction and simplification of the tail. Reconstructions modified from Unwin,15 Wellnhofer,16 and Witton.1 These are not to scale but are all set to a uniform torso length.
See also Figures S1–S8 and S11 and Table S1.Hone, David William Elliott; Fitch, Adam; Selzer, Stefan; Lauer, René; Lauer, Bruce
A new and large monofenestratan reveals the evolutionary transition to the pterodactyloid pterosaurs Current Biology (2024) Doi: 10.1016/j.cub.2024.10.023
Copyright: © 2024 The authors.
Published by Elsevier Inc. Open access.
Reprinted under a Creative Commons Attribution 4.0 International license (CC BY 4.0)
Refuting Creationism - How 70% of the Mediterranean Sea Was Lost 5.5 Million Years Before 'Creation Week'
How 70% of the Mediterranean Sea was lost 5.5 million years ago | CNRS

In the first phase, salt accumulated in a Mediterranean Basin filled with brine; in the second phase, salt accumulated in a Mediterranean completely isolated from the Atlantic Ocean, as a result of the significant drop in sea level in the western and eastern Mediterranean sub-basins.
This event was caused by land rising up under the straights of Gibraltar restricting inflow from the Atlantic and eventually closing it altogether, and, as water levels fell, the ridge between Sicily and the North African coast formed another barrier, effectively dividing the Mediterranean into two seas.
Saturday, 23 November 2024
New Book - The Failure Of Creationism: The Theory That New Was
While writing my previous book, Refuting Creationism: Why Creationism Fails In Both Its Science and Its Theology I realised what a monumental failure the creationist movement, in particular the Discovery Institute's 'Wedge Strategy', had been.
Just about every paper published in the fields of biology, palaeontology, cosmology and archaeology refuted just about every claim on which creationism rests, whether the claims of a young earth with the YEC obsession with a global flood, the supposed absence of 'transitional fossils', the proclaimed 'impossibility of 'life' or genetic information arising without a magic creator', or Michael J Behe's 'irreducible complexity'.
None of them have garnered the slightest degree of support on the scientific community and it holds as true today as it did When Behe was forced to admit under oath in the Kitzmiller case, "...there are no peer reviewed articles by anyone advocating for intelligent design supported by pertinent experiments or calculations which provide detailed rigorous accounts of how intelligent design of any biological system occurred."
Despite the regular repeated claims by creationists that the Theory of Evolution is a theory in crisis, about to be overthrown in scientific circles by Intelligent design theory, there is not the slightest indication of that happening. False witnessing remains the single most important tactic of the creationist movement as it seeks to fool ignorant people of the scientific validity of the childish superstition. Scientific support for Darwinian evolution remains as strong today as it was in 1991 when the Discovery Institute launched its campaign to confuse and misinform public and scientific opinion about the strength of the evidence for the TOE and the claimed 'gaps' and 'defects' in the theory.
The so-called 'controversy' was never anything of the sort and attempt to 'teach the controversy' and introduce Christian fundamentalism into public school science classes disguised as a legitimate science have been met with barrage of condemnations and detailed rebuttals from major science professional bodies and teaching estabishments.
The only success the Discovery Institute can claim, is in its campaign to confuse American public opinion about the level of support for creationism in biomedical science circles. While some 98-99% of biomedical scientists fully accept the TOE as the only explanation for humans in their modern form, American public opinion believes only some 66% do and that there is a debate to be had.
This new book, The Failure of Creationism: The Theory That Never Was, is an exposé of that failure with examples of recent scientific papers that implicitly refute creationism and appendices containing the absurd claims by creationists of the TOE imminent demise, the published opposition to teaching creationism in science classes by major scientific and educational bodies and the Discovery Institute's politically subversive 'Wedge Strategy'.
Monday, 11 November 2024
Refuting Creationism - Scientists Discover What Caused Earth's Climate Cycle To Change A million Years Before 'Creation Week'
Deep ocean clues to a million-year-old Ice Age puzzle revealed in new study – Woods Hole Oceanographic Institution
Like almost all of Earth's history, a sudden change in the cyclic pattern of climate change occurred in that long, pre-Creation period. To be precise, 700,000 to 1 million years ago the pattern of glaciation and interglacial warm spells changed from a 41,000 year cycle, due to changes in the degree of tilt in Earth's rotation (axial precession), to one of about 100,000 years with no obvious change in axial precession or external causes such as solar radiation. This is known to climatologists and geologists as the Mid-Pleistocene Transition (MPT)
Given the conviction of creationists that their putative designer god created Earth perfectly tuned for them to live on, it will probably be disturbing to learn that Earth's pattern of climate can change radically over the long term, and not caused by anthropogenic increases in greenhouse gases but by perfectly natural processes that don't need the interference of a magic deity to explain them. On top of this all happening before they believe Earth was created out of nothing as a small flat planet with a dome over it, there is much here for creationists to ignore and for their cult to lie about.
Sunday, 10 November 2024
Refuting Creationism - How The Grand Canyon Reveals Life On Earth 540 Million Years Before 'Creation Week',

The Grand Canyon often features in creationist disinformation websites because it needs to be explained away in terms of a history of Earth lasting only some 6-10,000 years and because it is easy to fool people who want to be fooled that it is somehow evidence if a global flood, and in particular how the water in the alleged flood ran away. Cult frauds also pretend the different rock layers in the canyon wall can all be explained in terms of sediment deposited during their god's supposed genocidal flood.
The truth, as usual with creationist claims, is nothing like the childish myth they like to pretend is real history. In fact, the walls of the Grand Canyon are a record of plate tectonics and climate change over hundreds of millions of years and mesh completely with what is known of Earth's history from other sources.
An indication of how creationists cult leaders are terrified of the information in the walls of the Grand Canyon, can be gauged from the notorious creationist purveyor of disinformation, Andrew Snelling's article on the creationists disinformation site, Answers in Genesis and the lengths he went to to obtain sample without disclosing exactly where he got them from, as related in this article in science. Snelling argued that it discriminated against his religion to require him to provide GPS coordinates of his samples! Clearly, Snelling believes his religion requires his 'science' to lack precision and reproducibility in case someone else tried to replicate his measurements and finds his to be bogus.
Snelling was subsequently given permission to collect samples under supervision and then wrote up his findings to try to explain away the fact that his findings didn't conform to his YEC preconceptions. His excuses include the creationists go-to excuse - the unsubstantiated claim that the uniformly old age of the rock he obtained must be because radioactive decay rates used to be different by several orders of magnitude!
One of Snellings stated objectives was to prove that the deformed Tapeats sandstone deposits, which he assures his readers are not fractured, despite the fact that photographs show fractures, were soft when deformed. He mentions this early in his article but then quietly drops the subject, presumably because his findings contradict his claim.
His findings are soundly refuted here.
Clearly, it is important to creationist cult leaders that their dupes are badly misinformed about the date of the rocks in the walls of the Grand Canyon, and it is obvious why, as this paper by geologists from Utah State University, together with colleagues from the University of New Mexico, Boise State University, Idaho, the University of Las Vegas, Nevada and the Denver Museum of Nature & Science, Denver, Colorado, shows. The rocks at the bottom of the canyon are from the Cambrian, 540 million years before creationists dogma says Earth was made from nothing by magic.
Other rocks map exactly onto what is known of changes in sea level and climate due to plate tectonics and how the canyon itself was carved into those strata is fully explainable in terms of erosion by a river flowing over a river bed that was rising slowly due to forces beneath Earth's crust over a period of millions of years.
Perhaps the most embarrassing thing for creationists is the thing they normally avoid like the plague - the famous Horseshoe bend, which requires their credulous dupes to believe a raging torrent of water, for no apparent reason, changed direction by over 180 degrees and headed back the way it came, when raging torrents of water are notoriously uni-directional.
And why on Earth anyone would imagine the water from a global flood would flow through a canyon in the middle of North America into the Pacific Ocean is anyone's guess and not something Andrew Snelling or any other creationist apologist has ever attempted to explain, simply leaving it to their parochial and culturally chauvinistic dupes to assume that anything important that happened in world history must have happened in the USA.
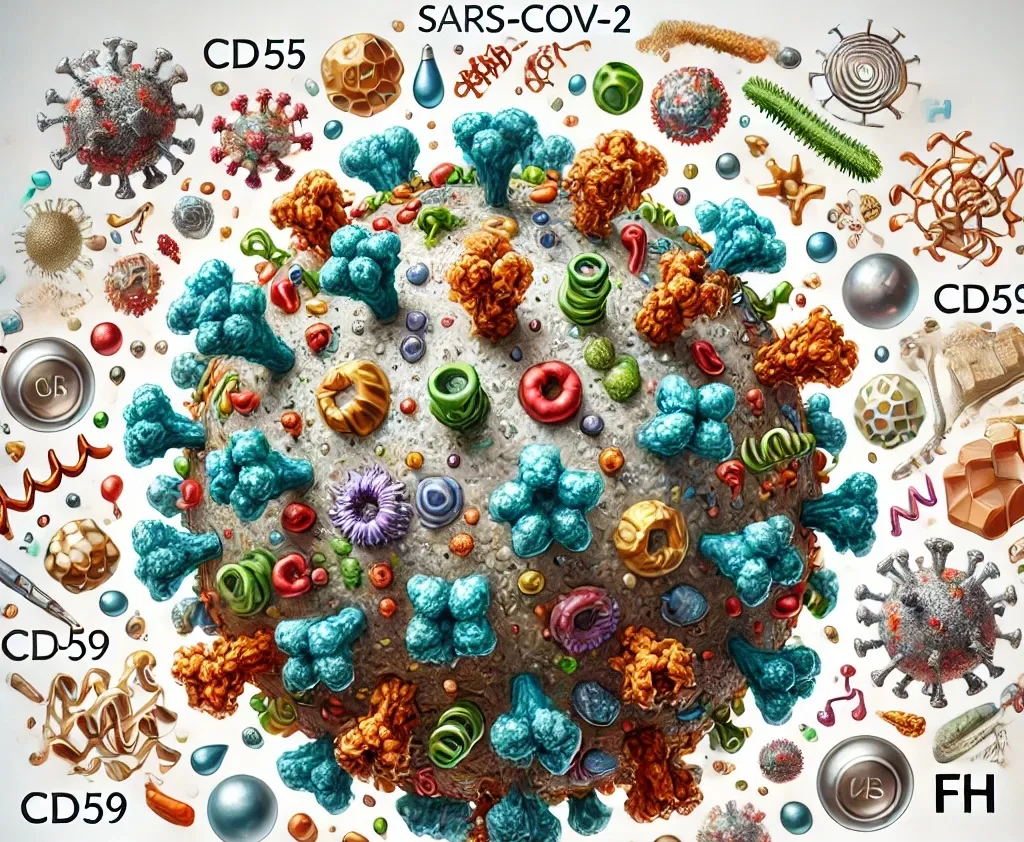
Malevolent Designer News - How The SARS-CoV-2 Virus Steals Proteins From Our Immune System To Protect Itself
SARS-CoV-2 “steals” our proteins to protect itself from the immune system
Although COVD-19 has been mostly brought under control by medical science and the vaccination campaign, it still kills thousands of people a year, but nowhere near the volume of deaths during the initial wave when world-wide health services came close to collapse and economies were on the point of ruin.
But there is still much to learn about why it was so virulent and successful.
To an admirer of creationism’s divine malevolence it must have seemed like a triumph of design, as it filled hospitals, killed millions and wrecked economies, helped by its supporters in the evangelical Christian churches who opposed measures to mitigate the worse effect of the virus, and then opposed the vaccination campaign with lies, scare tactics and the most infantile conspiracy theories imaginable, to help ensure the virus got to as many people as possible.
Now, a team of researcher from the Medical University of Vienna together with colleagues from the Medical University of Innsbruck have discovered how the virus protects itself from the immune system creationists believe their putative intelligent designer designed to protect us from the virus’s and other pathogens it designs to make us sick, would grace the pages of another 'intelligent design' polemic by Michael J. Behe and his Deception Institute. It depends on several components of a system being present in a classic 'irreducibly complex' system that creationists wave around as 'proof' that the locally-popular creator god is real because they can't understand how it could have evolved.
Saturday, 9 November 2024
Refuting Creationism - What Did The Denisovans Ever Do For Us?
New insights into the Denisovans – the new hominin group that interbred with modern day humans - News & Events | Trinity College Dublin
In marked contrast to the childish creationist notion of a single founder couple being magically created without ancestors 6-10,000 years ago, evidence is growing that one ancestral species that contributes some of its DNA to modern non-African humans, the Denisovans, were once widespread especially in Southeast Asia and may have reached South America, or at least people carrying some Denisovan DNA may have done, but not via the traditional route - Siberia, Beringia and Alaska - followed by later Homo sapiens.
My understanding is that they and Neanderthals were most likely direct descendants of H. erectus that migrated out of Africa some 2 million years ago and gave rise to the Denisovans in Eastern Eurasia and Neanderthals in Western Eurasia. These two then interbreed with the H. sapiens migrants as they came up out of Africa and spread throughout Eurasia and down to Melanesia, Austronesia and Oceania.
So, rather than a single ancestral couple magically created out of dirt, without ancestors, as creationists believe, modern non-African humans don't have an ancestral couple, they don't even have a single ancestral species but are the result of hybridization between at least three ancestral species.
There is also evidence, according to two researchers from Trinity College, Dublin, Ireland, that there may have been several regional populations of Denisovans, each of which contributed to the Homo sapiens genome at different times. As with other hominin species, they were diversifying as they spread in what may have been the beginnings of classical allopatric speciation.
The Denisovan DNA that was retained by H. sapiens as they migrated into the different environments in Asia was that which gave them an advantage, such as the ability to survive in the low oxygen partial pressure of the Tibetan Plateau - something that the Tibetans have inherited - immunity to certain endemic pathogens and an improved ability to keep their body temperature up during cold weather by burning stored body fats - something that Innuits have inherited.
Friday, 8 November 2024
Refuting Creationism - How Bird's and Bat's Wings Evolved

Unlike an intelligent designer, the process of evolution can't go back to basic and start again. It is normally an additive process that has no control over what it has to work with and simply refines and improves on what is there. That's not to say new structures can't evolve but they do so by enlarging or remodelling something that was already there - the membrane of a bat's wing, for example is the webbing that exists in the tetrapod embryo between the fingers and toes, while the feathers of a bird's wing are highly modified scales. Both those structures evolved out of tissues that were already there. It would have been impossible for a bat to grow wing feathers instead of a membrane, for example, because the earliest mammals had lost their scales and evolved fur.
But of course, that would not have been a problem for an omnipotent intelligent designer who, having designed one wing would not need to set about designing another way to do the same thing.
So, constrained as evolution was by what it could use, it's not really surprising that birds and bats evolved on two different trajectories, with the only thing in common being flight (and of course the basic vertebrate skeletal body plan).